Application of human recombination protein PDCD5 in preparing tumor chemotherapy sensitizing medicine
A technology of human recombination and protein, which is applied in the direction of anti-tumor drugs, peptide/protein components, drug combinations, etc., and can solve the problems of limiting chemotherapy and the toxic effects of chemotherapy drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, construction PDCD5 Escherichia coli expression plasmid
[0032] According to the cDNA and protein sequences of the PDCD5 coding region (see Chinese patent application: ZL98101869.6, publication number CN1218833A), PDCD5 was directional inserted into the pET30a vector (purchased from Novagen) using the NdeI and HindIII sites to obtain the pET30a-PDCD5 expression vector. After the correctness of the plasmid was verified by sequencing, the BL21 strain was transformed.
Embodiment 2
[0033] Embodiment 2, expression and purification of PDCD5 in Escherichia coli
[0034] After the ET30a-PDCD5 expression vector was transformed into BL21(DE3) Escherichia coli and amplified, 1mMIPTG (isopropyl-β-D-thiogalactoside) (Sigma-Aldrich) induced the expression of human recombinant PDCD5 protein, which was anion-exchanged with DEAE Chromatography (Amersham Pharmacia Biotech) and hydrophobic chromatography (Amersham Pharmacia Biotech) to obtain PDCD5 purified protein. used in the following experiments.
[0035] The results are shown in FIG. 1 , by densitometric scanning, the expression level of the target protein PDCD5 is about 8%. Most of the protein was expressed in the supernatant form. As shown in Figure 2, by exploring and optimizing the separation and purification conditions, after two-step purification by ion exchange and hydrophobic chromatography, human recombinant PDCD5 protein with a purity greater than 95% at the electrophoretic level can be obtained.
Embodiment 3
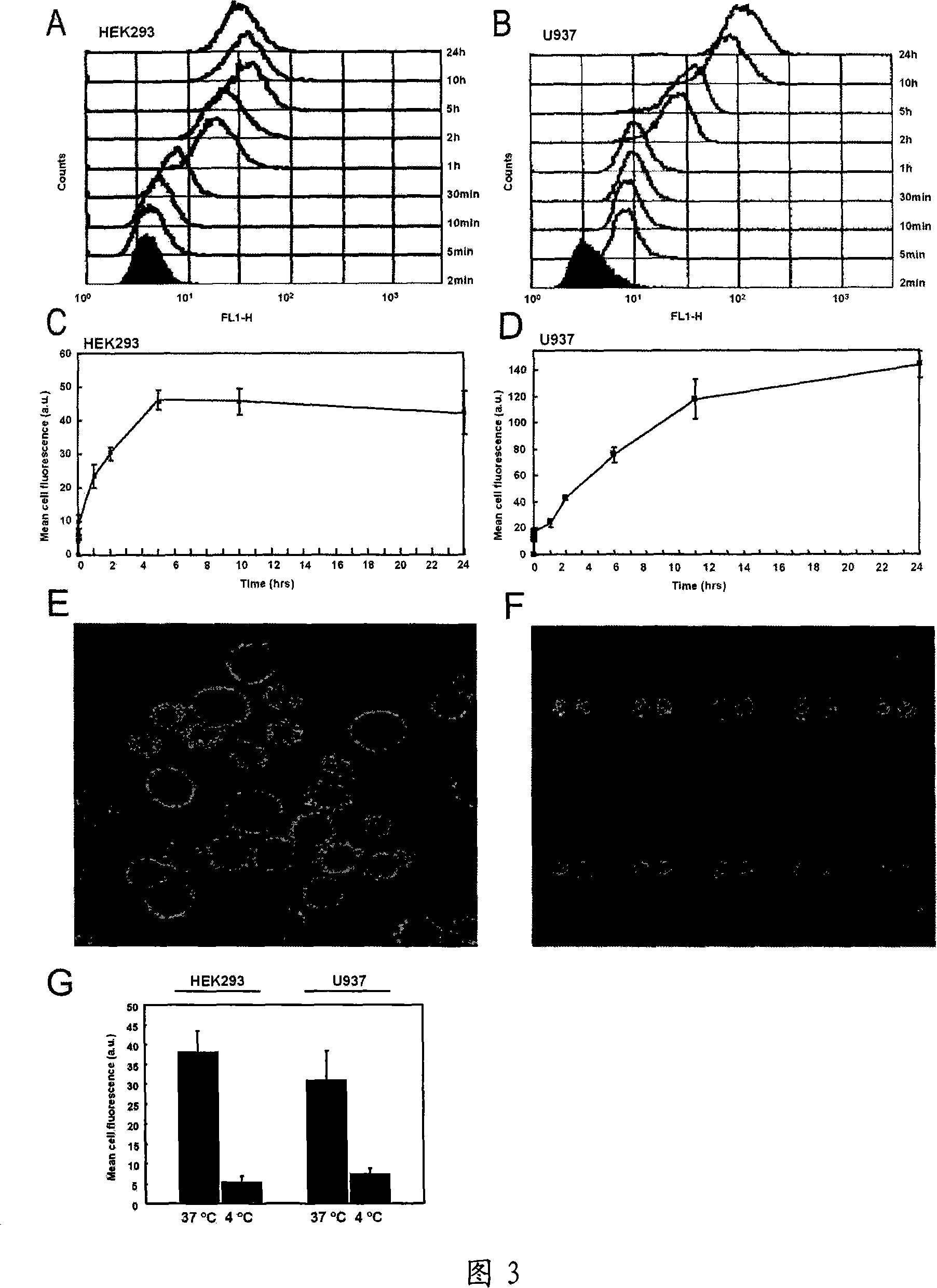
[0036] Embodiment 3, PDCD5 internalization kinetics
[0037] HEK293 and U937 cells (purchased from ATCC) were cultured in RPMI containing 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, USA), 100 U / ml penicillin, 100 μg / ml streptomycin, 2 mM L-glutamic acid 1640 (Life Technologies, USA). HEK293 cells are an adherent-growing cell expressing caveolin-1, and U937 cells are a suspension-growing cell that does not express caveolin-1.
[0038] Human recombinant PDCD5 protein was labeled with fluorescein isothiocyanate (FITC) in 0.025M carbonate buffer (pH9.0), and then purified by Sephadex G25 to remove free FITC , to obtain PDCD5-FITC protein.
[0039] Flow cytometric analysis of the internalization of FITC-labeled PDCD5 recombinant protein: Culture HEK293 and U937 cells to 70% in 24-well plates, and then incubate with 5 μg / ml recombinant PDCD5-FITC. The incubation time points are shown in the figure (Figure 3) . The cells were washed twice with PBS, treated with trypsi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com