Aluminate luminescent material and preparation method thereof
A luminescent material, aluminate technology, applied in luminescent materials, chemical instruments and methods, alumina/aluminum hydroxide and other directions, can solve the problems of high synthesis temperature, difficult mixing of raw materials, unsatisfactory particle crystal shape, etc. The effect of high efficiency, short preparation period and long afterglow time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
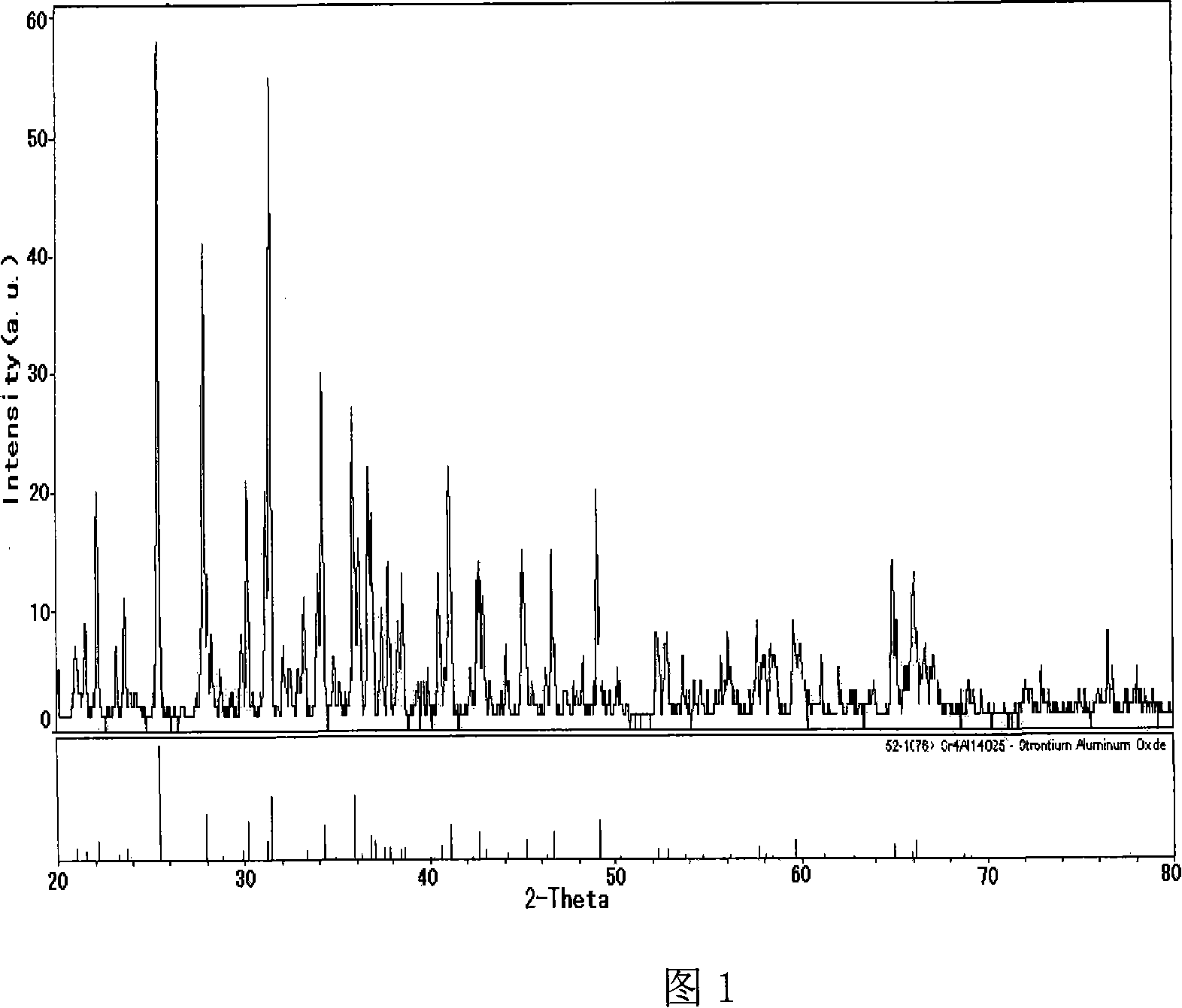
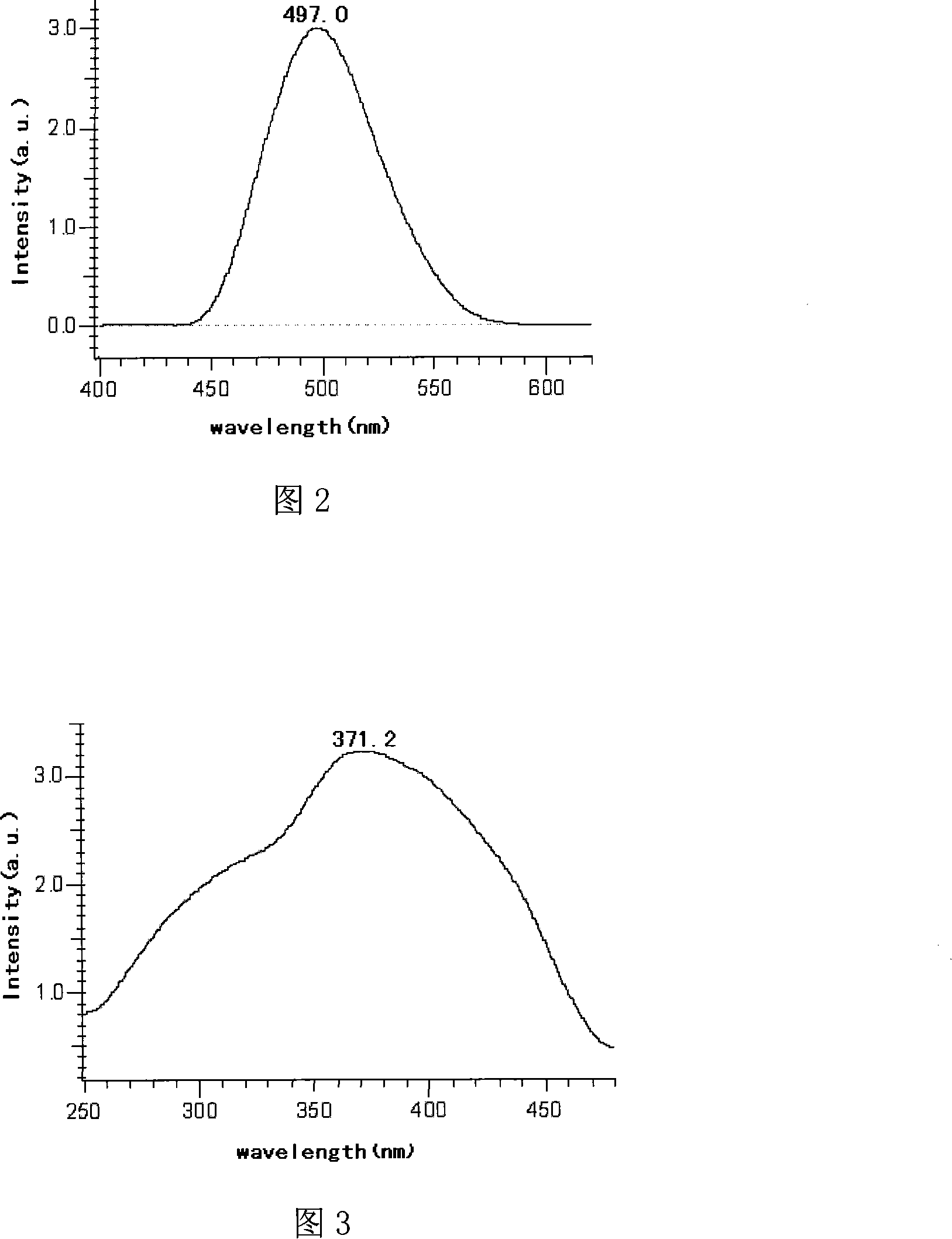
Image
Examples
Embodiment 1
[0016] 0.98mol / l AlCl 3 Solution 50ml, 0.1mol / l Eu(NO 3 ) 3 Solution 2.8ml, 0.08984mol / l Dy(NO 3 ) 3 Solution 3.1ml, SrO 1.4506g, NH 4 HCO 3 20g, H 3 BO 3 0.0216g.
[0017] The above AlCl 3 solution with Eu(NO 3 ) 3 solution, Dy(NO 3 ) 3 The solution is mixed in a container, 20g of NH 4 HCO 3 Dubbed a 150ml solution and put it into another container. Use a peristaltic pump to inject the solutions in the two containers into the reaction vessel simultaneously (there is a small amount of NH in the reaction vessel 4 HCO 3 solution as the reaction bottom solution), heated to 50°C in a water bath, stirred while heating, and the reaction continued for two hours. Suction filtration and washing of the gel obtained by the reaction were repeated several times until CO 3 2- was removed. Disperse the gel with deionized water, add the above-ground SrO into the dispersed gel, oscillate ultrasonically, mix evenly, and then transfer to a hydrothermal kettle for hydrotherma...
Embodiment 2
[0020] 1.026mol / l AlCl 3 Solution 68ml, 0.095mol / l Eu(NO 3 ) 3 Solution 4.2ml, 0.08984mol / l Dy(NO 3 ) 3 Solution 4.5ml, SrO 2.0724g, NH 4 HCO 3 21g, H 3 BO 3 0.0312g.
[0021] Take the above AlCl 3 solution with Eu(NO 3 ) 3 solution, Dy(NO 3 ) 3 The solution was mixed in a container, 21g of NH 4 HCO 3 Dubbed a 150ml solution and put it into another container. Use a peristaltic pump to inject the solutions in the two containers into the reaction vessel simultaneously (there is a small amount of NH in the reaction vessel 4 HCO 3 solution as the reaction bottom solution), heated in a water bath at 80°C, stirred while heating, and the reaction continued for two hours. Suction filtration and washing of the gel obtained by the reaction were repeated several times until CO 3 2- was removed. Disperse the gel with deionized water, add the ground SrO to the dispersed gel, oscillate ultrasonically, mix evenly, and then transfer to a hydrothermal kettle for hydrotherma...
Embodiment 3
[0024] 5.023mol / l AlCl 3 Solution 100ml, 0.095mol / l Eu(NO 3 ) 3 Solution 30ml, 0.08984mol / l Dy(NO 3 ) 3 Solution 32ml, SrO 14.8709g, NH 4 HCO 3 100g, H 3 BO 3 0.2230g.
[0025] Take the above AlCl 3 solution with Eu(NO 3 ) 3 solution, Dy(NO 3 ) 3 The solution is mixed in a container, 100g of NH 4 HCO 3 Dubbed a 250ml solution and put it into another container. Use a peristaltic pump to inject the solutions in the two containers into the reaction vessel simultaneously (there is a small amount of NH in the beaker 4 HCO 3 solution as the reaction bottom solution), heated in a water bath at 80°C, stirred while heating, and the reaction continued for two hours. Suction filtration and washing of the gel obtained by the reaction were repeated several times until CO 3 2- was removed. Disperse the gel with deionized water, add the above-ground SrO into the dispersed gel, oscillate ultrasonically, mix evenly, then transfer to a hydrothermal kettle, and conduct a hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com