Process for the preparation of diisopinocampheylchloroborane
A technology for pinenyl chloroborane and diisopine, which is applied in the field of preparing diisopinepine pinyl chloroborane from Lewis acid, can solve problems such as high risk, unsafe industrial production, and toxicity, and reduce production costs. , the effect of reducing hidden dangers in production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
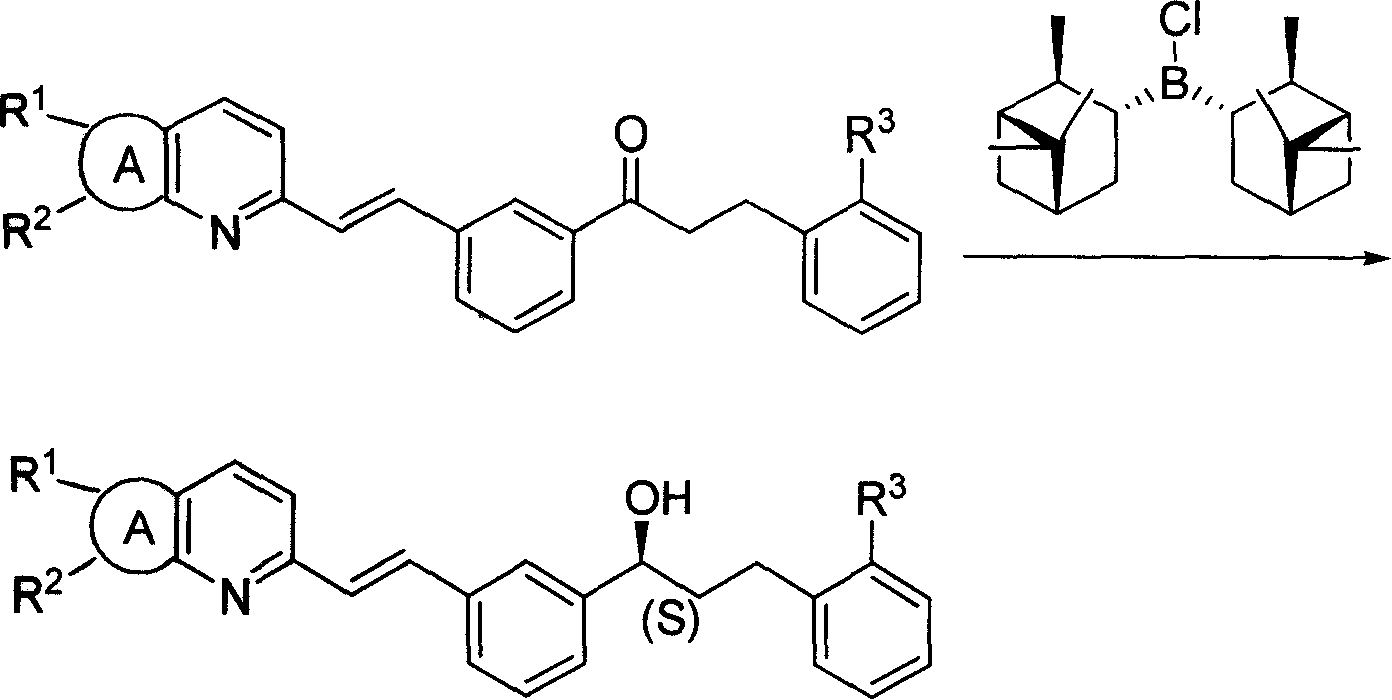
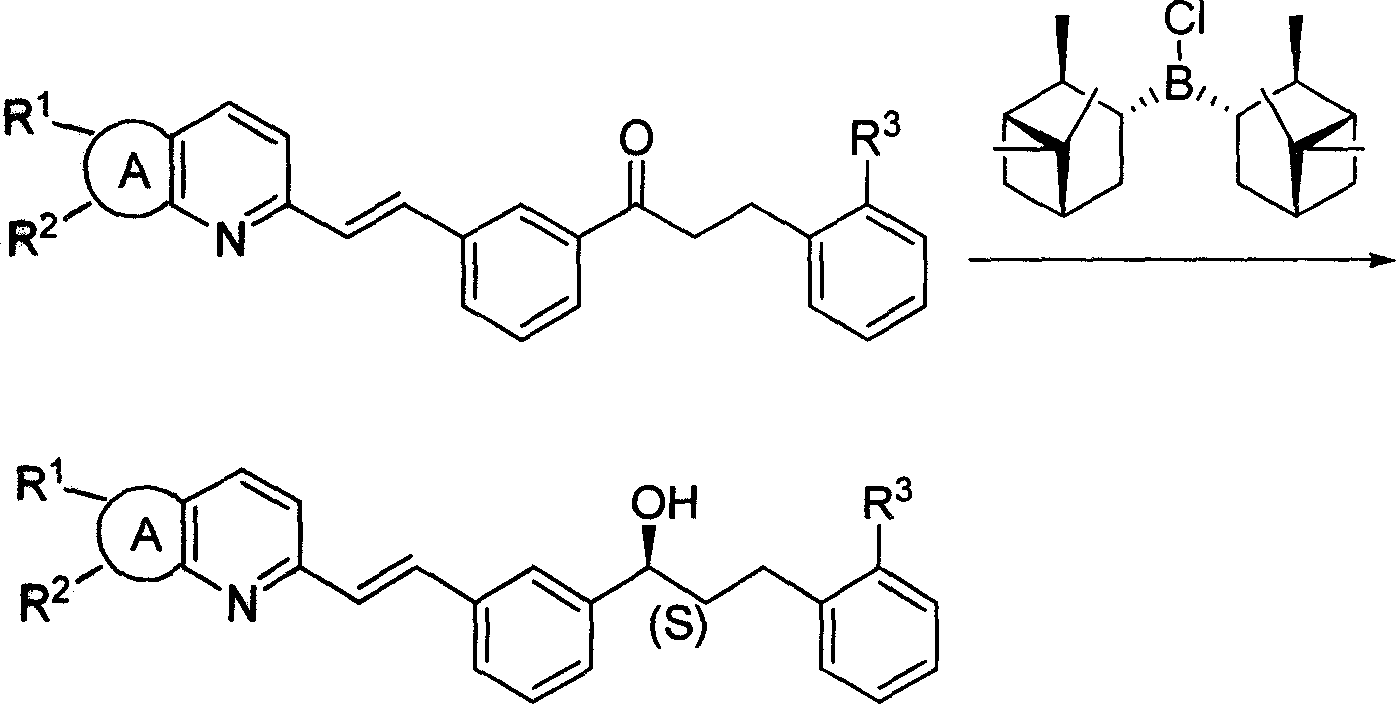
[0034] The preparation of diisopine pinocampyl chloride borane:
[0035] 1.14g (30mmol) NaBH 4 Add to the three-necked bottle, add 30ml ethylene glycol dimethyl ether, 28.8ml (180mmol) (1R)-(+)-α-pinene (ee.50%; [α] 20 D , neat: +27.4°). Add 7.5ml (60mmol) BF dropwise under stirring at -10°C 3 ·Et 2 O, about 30 minutes to drop. Raised to 0°C and stirred for 4 hours (the liquid was a milky white viscous liquid). Add 7.4g (35.5mmol) PCl at 0°C 5 , raised to 100°C and stirred for 3 hours, the solution was left to cool naturally, and then directly entered into the next step of reaction. The molar concentration of diisopinocampylchloroborane is 1M.
[0036] Chiral reduction:
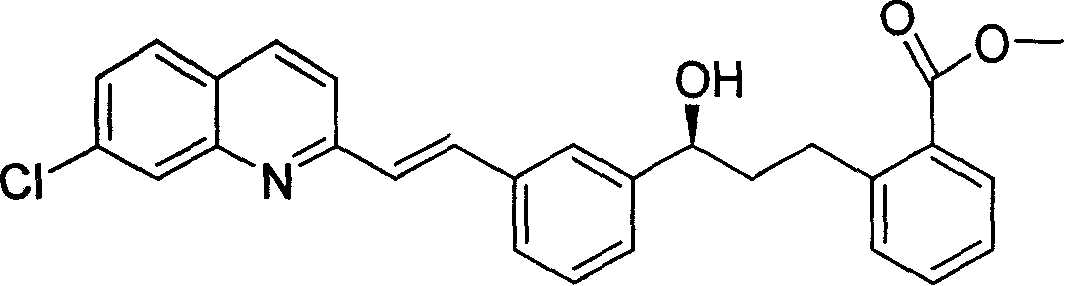
[0037] Dissolve 1.5 g (3.3 mmol) of methyl 2-(3-(3-(2-(7-chloro-2-quinolyl) vinyl) phenyl) 3-oxopropyl) benzoate in 30 ml of THF , Add the obtained catalyst solution under stirring at -15°C, and stop the refrigeration after stirring for 8 hours. Slowly add 31% K in a water bath 2 CO 3 The aqueous...
Embodiment 2
[0039] The preparation of diisopine pinocampyl chloride borane:
[0040] 1.14g (30mmol) NaBH 4 Add to the three-necked flask, add 30ml ethylene glycol dimethyl ether, 19.2ml (120mmol) (1R)-(+)-α-pinene (ee.65%; [α] 20 D , neat: +27.4°). Add 6ml (48mmol) BF dropwise under stirring at -10°C 3 ·Et 2 O, about 30 minutes to drop. Raised to 0°C and stirred for 4 hours (the liquid was a milky white viscous liquid). Add 7.4g (35.5mmol) PCl at 0°C 5 , raised to 80°C and stirred for 1 hour, the solution was left to cool naturally, and then directly entered into the next step of reaction. The molar concentration of diisopinocampylchloroborane is 1M.
[0041] Chiral reduction:
[0042] Dissolve 5 g (11 mmol) of methyl 2-(3-(3-(2-(7-chloro-2-quinolyl) vinyl) phenyl) 3-oxopropyl) benzoate in 85 ml THF, - The obtained catalyst solution was added under stirring at 16°C, and after stirring for 8 hours, the refrigeration was stopped. Slowly add 31% K in a water bath 2 CO 3 The aque...
Embodiment 3
[0044] The preparation of diisopine pinocampyl chloride borane:
[0045] 760mg (20mmol) NaBH 4 Add in the three-necked flask, add 20ml ethylene glycol dimethyl ether, 6.4ml (40mmol) (1s)-(-)-α-pinene (ee.>80%; [α] 20 D : -45°). Add 4ml (32mmol) BF dropwise under stirring at -10°C 3 ·Et 2 O, about 30 minutes to drop. Warm to 0°C and stir for 5 hours. Add 4.9g (24mmol) PCl at 0°C 5, raised to 85°C and stirred for 0.5 hours, the solution was left to cool naturally, and then it could directly enter the next step of reaction. Molar concentration of diisopinocampylchloroborane at 1 M chiral reduction:
[0046] 9g (20mmol) 2-(3-(3-(2-(7-chloro-2-quinolyl) vinyl) phenyl) 3-oxopropyl) methyl benzoate was dissolved in 100ml THF,- The obtained catalyst solution was added under stirring at 24°C, and after stirring for 9 hours, the refrigeration was stopped. Slowly add 31% K in a water bath 2 CO 3 The aqueous solution is about 40ml, and the pH is neutralized to 7. The organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com