Anti-cancer drug compounds and method for synthesizing same
An anti-cancer drug and compound technology, applied in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., can solve problems such as no substantial breakthroughs, inhibit the activity of Raf and VEGFR protein kinases, induce tumor cell apoptosis, Inhibitory effect of human liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
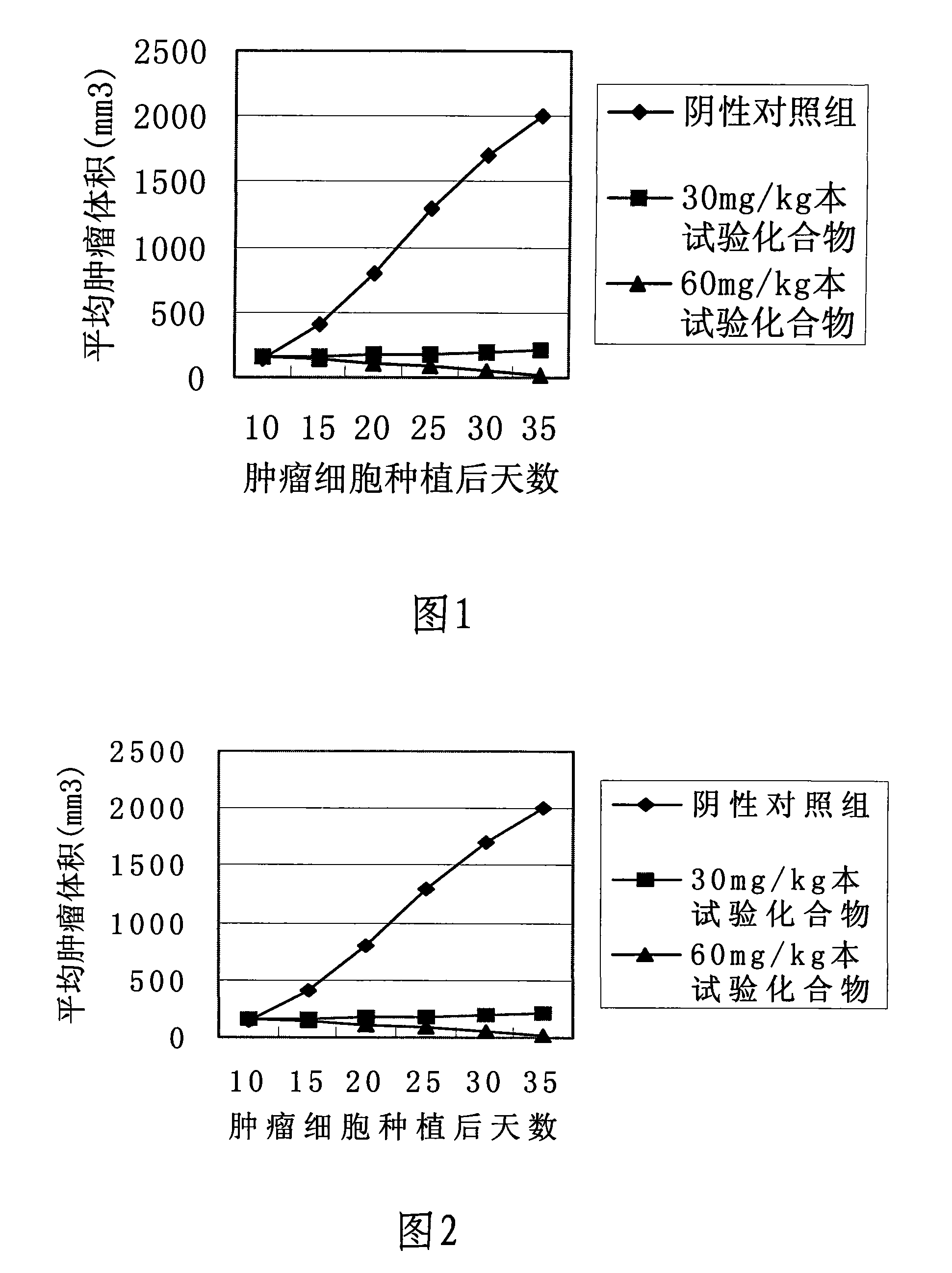
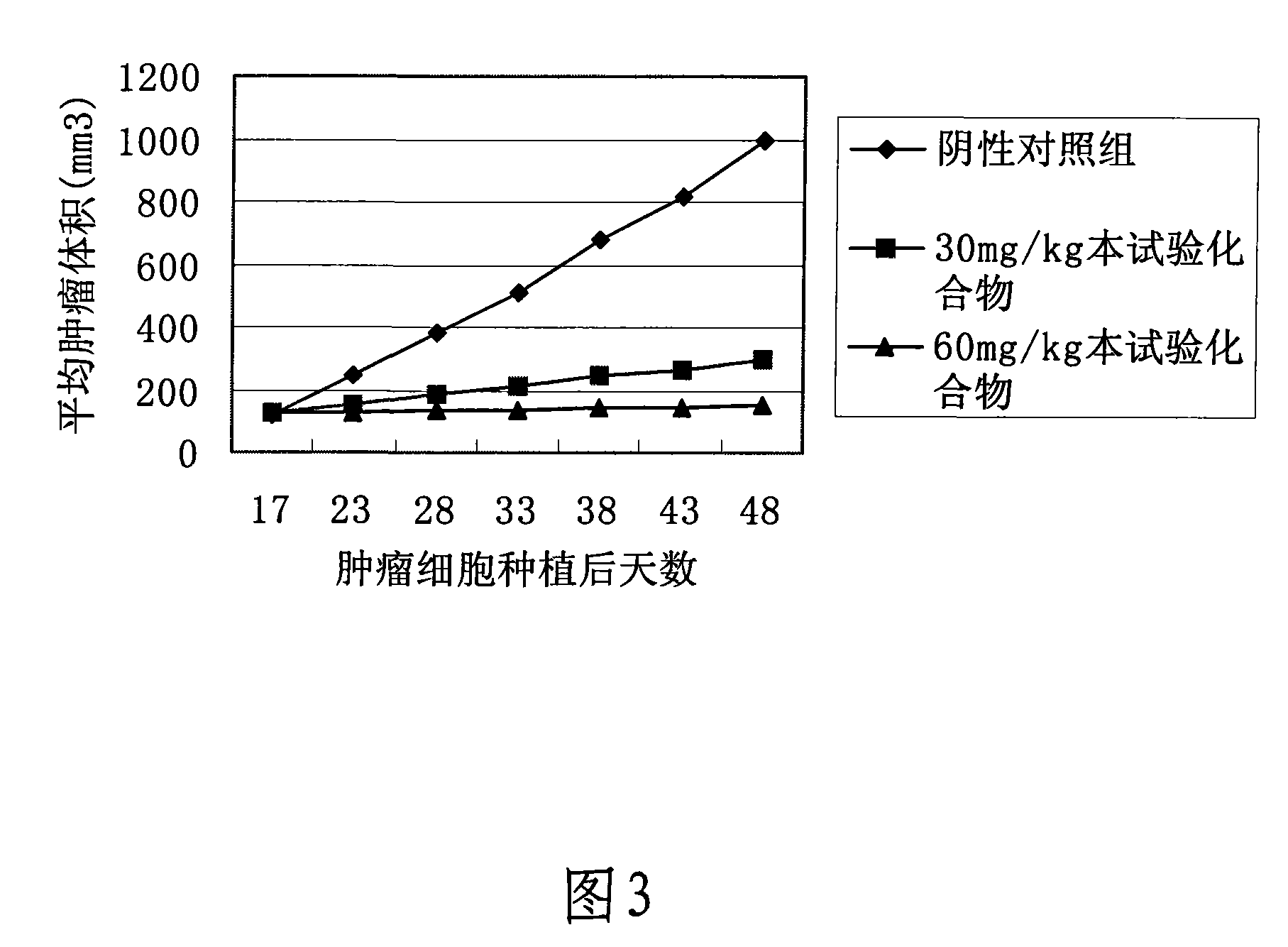
Image
Examples
Embodiment 1
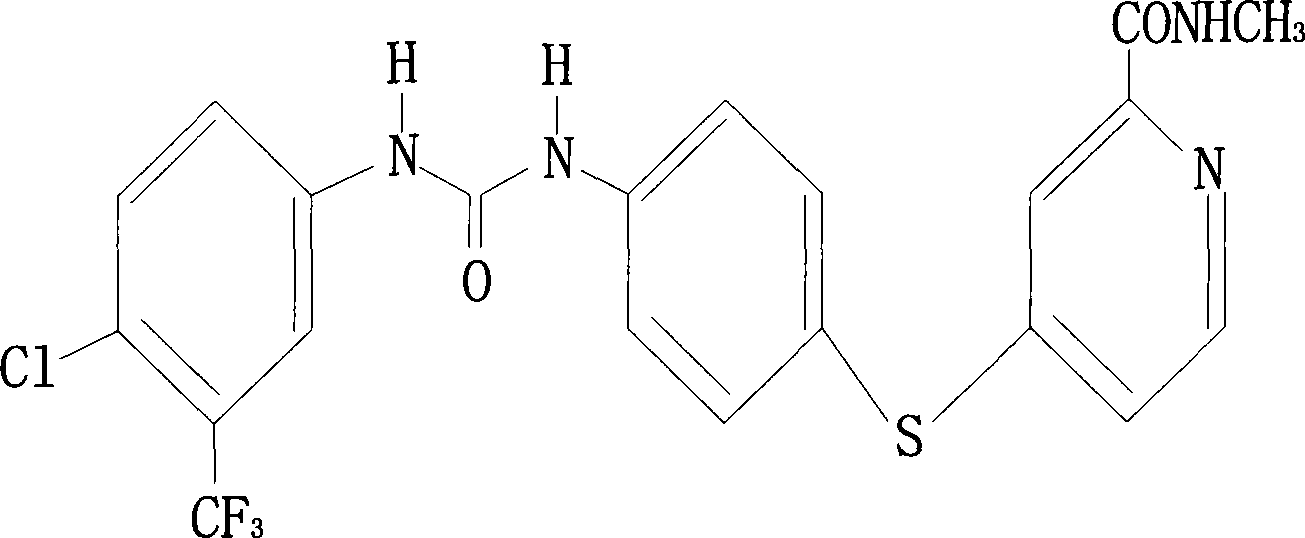
[0023] An anticancer drug compound, compound N-[4-chloro-3-(trifluoromethyl)phenyl]-{[4-(N-methyl-carbamoyl)(4-pyridylthio)phenyl ]amino}-carboxamide, its chemical structural formula is:
[0024]
[0025] Proton nuclear magnetic resonance spectrum (H NMR) analysis result (300MHz, DMSO-d 6 ): δ2.77 (d, J=4.8, 3H, -NHCH 3 ); 7.16 (m, 3H, aromatic cluster ring); 7.37 (d, J = 2.5Hz, 1H, aromatic cluster ring); 7.62 (m, 4H, aromatic cluster ring); 8.11 (d, J = 2.5Hz, 1H , aromatic cluster ring); 8.49 (d, J=5.5Hz, 1H, aromatic cluster ring); 8.77 (brd, 1H, -NHCH 3 ); 8.89(s, 1H, -NHCO-); 9.12(s, 1H, -NHCO-); chemical ionization mass spectrometry and high resolution mass spectrometry confirmation (C 21h 16 N 4 CIF 3 SO 2 ) has a molecular weight of 434.5.
[0026] A synthetic method of an anticancer drug compound, the synthetic steps are as follows:
[0027] The first step: the preparation of 4-chloropyridine-2-carbonyl chloride
[0028] At a temperature of 50°C and fill...
Embodiment 2
[0036] A synthetic method of an anticancer drug compound, the synthetic steps are as follows:
[0037] The first step: the preparation of 4-chloropyridine-2-carbonyl chloride
[0038] At a temperature of 50°C and filled with argon, slowly add 8 ml of N,N,-dimethylformamide to 200 ml of thionyl chloride, stir for 20 minutes, and then slowly add 480 grams of it within 40 minutes. 2-Pyridinecarboxylic acid, green at first, then orange and finally purple, the reaction solution was heated to 85°C and a large amount of sulfur dioxide was produced, and a yellow solid precipitated after 18 hours of reaction, the mixture was cooled to room temperature at 25°C, and then Dilute with 600 ml of toluene and concentrate to 250 ml. This process is repeated 3 times. Finally, the product is concentrated to 95% dry state. At this time, the mixture is transferred to a filter for filtration, washed with 60 ml of toluene, and finally vacuumed for 5 hours. Obtain 100 grams of 4-chloropyridine-2-car...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com