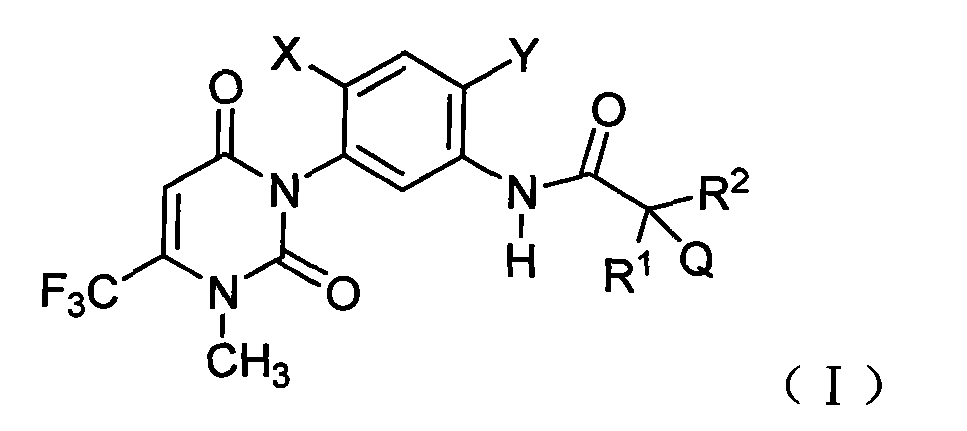
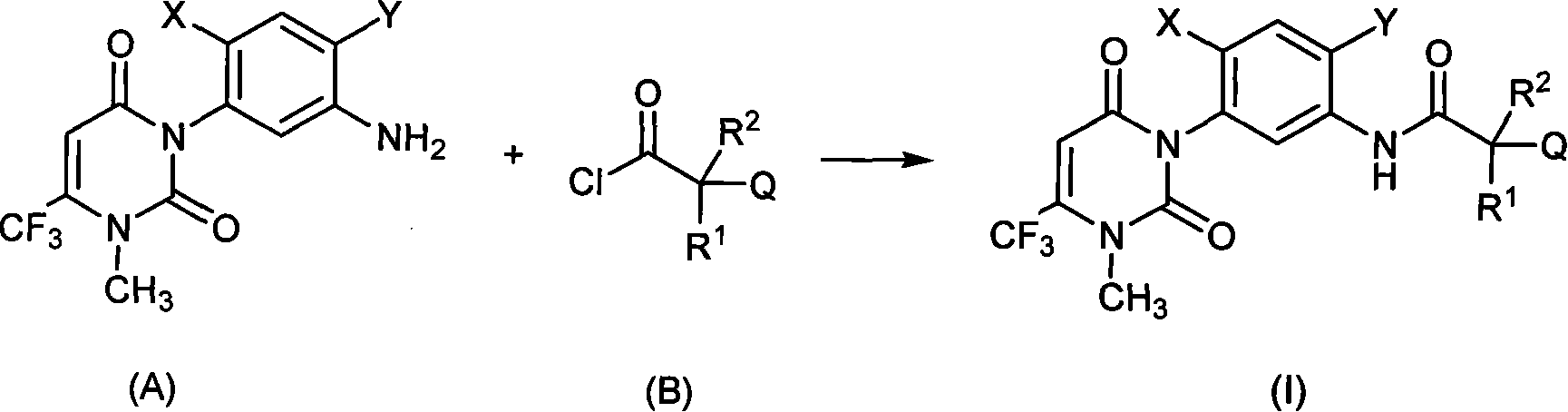
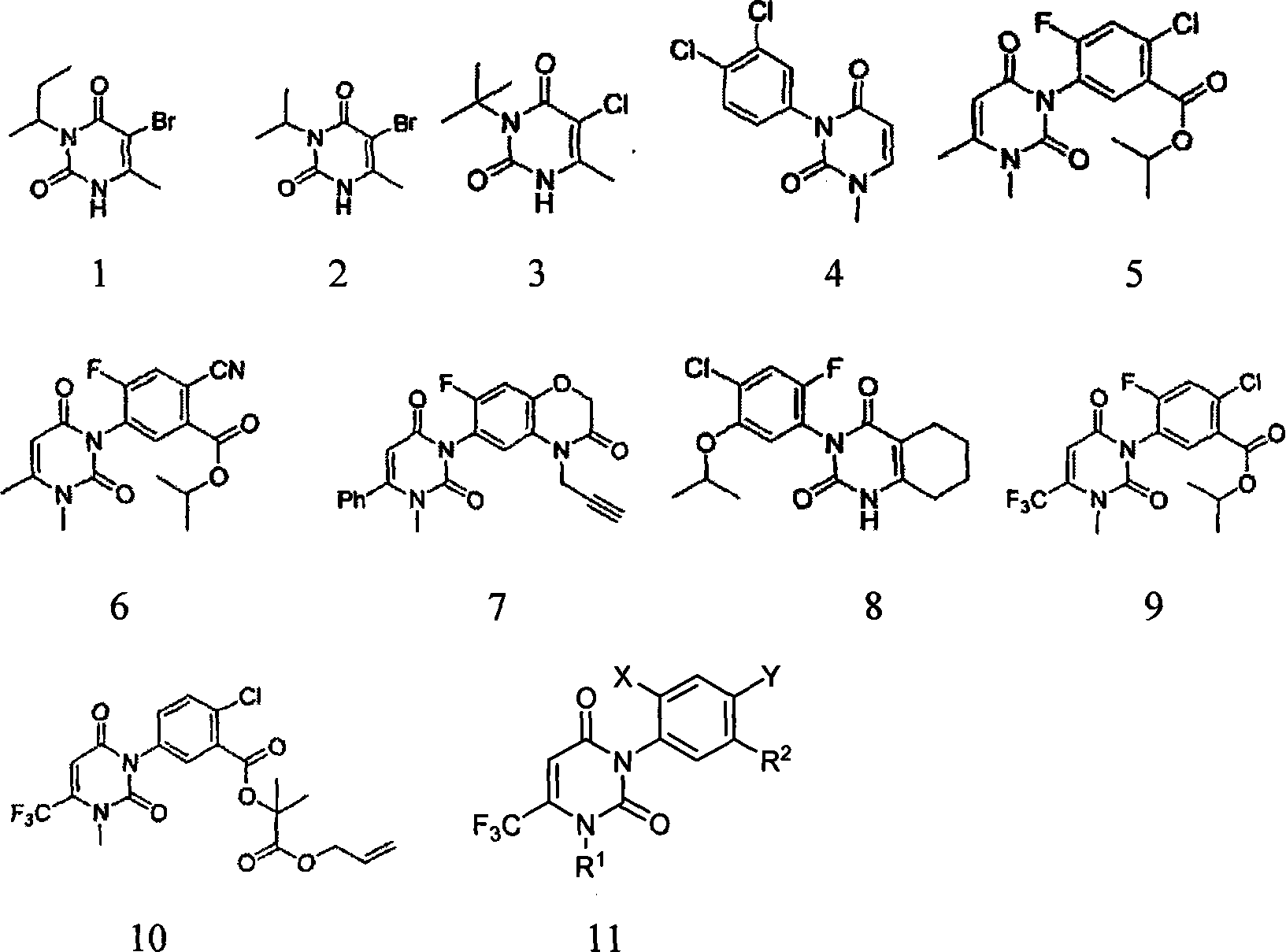
Uracil weeding compounds introducing amide structure
A technology of uracil and compounds, which is applied in the field of uracil herbicidal compounds and can solve problems such as poor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: 3-chloro-N-(2-chloro-4-fluoro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-2,3-dihydropyrimidine Preparation of -1(6H)-yl)phenyl)-2,2-dimethylpropanamide (compound 1-05).
[0084] 1.35 g (4.0 mmol) of intermediate 3-(5-amino-4-chloro-2-fluorophenyl)-1-methyl-6-(trifluoromethyl)pyrimidine-2 was added to a 100 mL round bottom flask, 4(1H,3H)-diketone, 30mL dichloroethane and 0.45g (4.4mmol) triethylamine, magnetically stirred, under ice-water cooling, slowly added dropwise 0.62g (4.0mmol) chloropivaloyl chloride, Dropwise reaction at room temperature for 2h. 100 mL of water was added to the reaction solution, and the organic layer was separated. The organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, precipitated under reduced pressure, and subjected to silica gel column chromatography to obtain 1.34 g of light yellow solid 3-chloro-N -(2-Chloro-4-fluoro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-2,3-dihydrop...
Embodiment 2
[0086] Example 2: N-(2-chloro-4-fluoro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-2,3-dihydropyrimidine-1(6H )-yl)phenyl)-2-(3-fluorophenoxy)propionamide (compound 1-09).
[0087] 1.35 g (4.0 mmol) of intermediate 3-(5-amino-4-chloro-2-fluorophenyl)-1-methyl-6-(trifluoromethyl)pyrimidine-2 was added to a 100 mL round bottom flask, 4(1H,3H)-diketone, 30mL dichloroethane and 0.45g (4.4mmol) triethylamine, magnetically stirred, under ice water cooling, slowly drop 0.8g (4.0mmol) 2-m-fluorophenoxy Propionyl chloride, dropwise reaction at room temperature for 2h. 100mL of water was added to the reaction solution, and the organic layer was separated. The organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, precipitated under reduced pressure, and subjected to silica gel column chromatography to obtain 1.44g of a light yellow viscous substance, N- (2-Chloro-4-fluoro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-2,3-dihydropyrimidin-1...
Embodiment 3
[0089] Example 3: N-(4-chloro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-2,3-dihydropyrimidin-1(6H)-yl) Preparation of phenyl)-2-(2,4-di-chlorophenoxy)propanamide (compound 1-21).
[0090] Into a 100 mL round bottom flask was added 1.28 g (4.0 mmol) of the intermediate 3-(5-amino-4-di-chloro-2-fluorophenyl)-1-methyl-6-(trifluoromethyl)pyrimidine- 2,4(1H,3H)-diketone, 30mL dichloroethane and 0.45g (4.4mmol) triethylamine, magnetically stirred, under ice-water cooling, slowly add 1.01g (4.0mmol) 2-(2 , 4-di-chlorophenoxy) propionyl chloride, dropwise reaction at room temperature for 2h. 100 mL of water was added to the reaction solution, and the organic layer was separated. The organic layer was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, precipitated under reduced pressure, and subjected to silica gel column chromatography to obtain 1.64 g of light yellow solid N-(4- Chloro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-2,3-dihydropyrimi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com