Pyrimidinoxy salicylic acid derivative
A technology for pyrimidyloxysalicylic acid and derivatives, which is applied in the field of pyrimidyloxysalicylic acid derivatives, can solve problems such as selectivity and limited application range of crops, and achieve good herbicidal effect, high herbicidal activity, and effective herbicidal activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
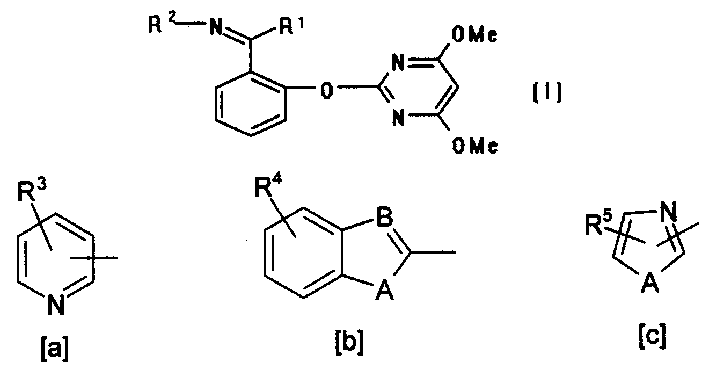
[0038] Synthesis Intermediate (IV)-1 R 1 =H, R 2 = 2-pyridyl
[0039] The 250ml round bottom flask is connected with a water separator, a condenser and a calcium chloride drying tube. 62.0g of salicylaldehyde, 47.0g of 2-aminopyridine, 100ml of toluene and a catalytic amount of p-toluenesulfonic acid are added to the bottle, electromagnetic stirring The temperature was raised and refluxed for 6 hours, the toluene was evaporated and then cooled, an orange solid was precipitated, which was recrystallized by 100 ml of petroleum ether-ethyl acetate with a molar ratio of 1:4 to obtain 80 g of orange crystals.
[0040] H 1 NMR: δ 13.47 (S, 1H); δ 9.45 (S, 1H); δ 6.94-8.92 (M, 8H).
[0041] MS: 198(M - ).
Synthetic example 2
[0043] Synthetic compound (I)-1 R 1 =H, R 2 = 2-pyridyl
[0044] After drying the reaction system to remove water, add 5.58 g of sodium hydride solid (55% paraffin wax suspension agent) and 100 ml of anhydrous tetrahydrofuran to a 1000 ml round bottom flask under the protection of nitrogen flow, and add dropwise under electromagnetic stirring at room temperature. Example 1 prepared 19.8 g (IV)-1 in 150 ml of anhydrous tetrahydrofuran solution. During this process, hydrogen gas was released, resulting in an orange-yellow suspension. Then, a solution of 21.8 g of 4,6-dimethoxy-2-methylsulfonylpyrimidine (V) in 500 ml of anhydrous tetrahydrofuran was added dropwise, and the mixture was stirred at room temperature overnight. After filtration, the mother liquor was concentrated, and recrystallized with tetrahydrofuran-petroleum ether at a molar ratio of 2:1 to obtain 20.5 g of yellow crystals.
[0045] H 1 NMR: δ 9.29 (S, 1H); δ 5.78 (S, 1H); δ 3.80 (S, 1H);
[0046] δ 6.48-8.45 (M, 8H...
Synthetic example 3
[0049] Synthesis Intermediate (IV)-12 R 1 =H, R 2 = 2-(6-methyl)benzothiazolyl
[0050] Add 1.22 g of salicylaldehyde, 40 ml of methanol, and 1.84 g of 2-amino-(6-chloro)benzothiazole to a 100 ml round-bottom flask. The temperature is raised and refluxed for 10 minutes under electromagnetic stirring, and then slowly cooled, orange crystals are precipitated, and filtered. 1.4 g of the product was obtained.
[0051] MS: 288(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com