Stabilizer for protein preparation comprising meglumine and use thereof
A kind of meglumine, protein technology, applied in the stabilizer of protein preparation containing meglumine and its utilization field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
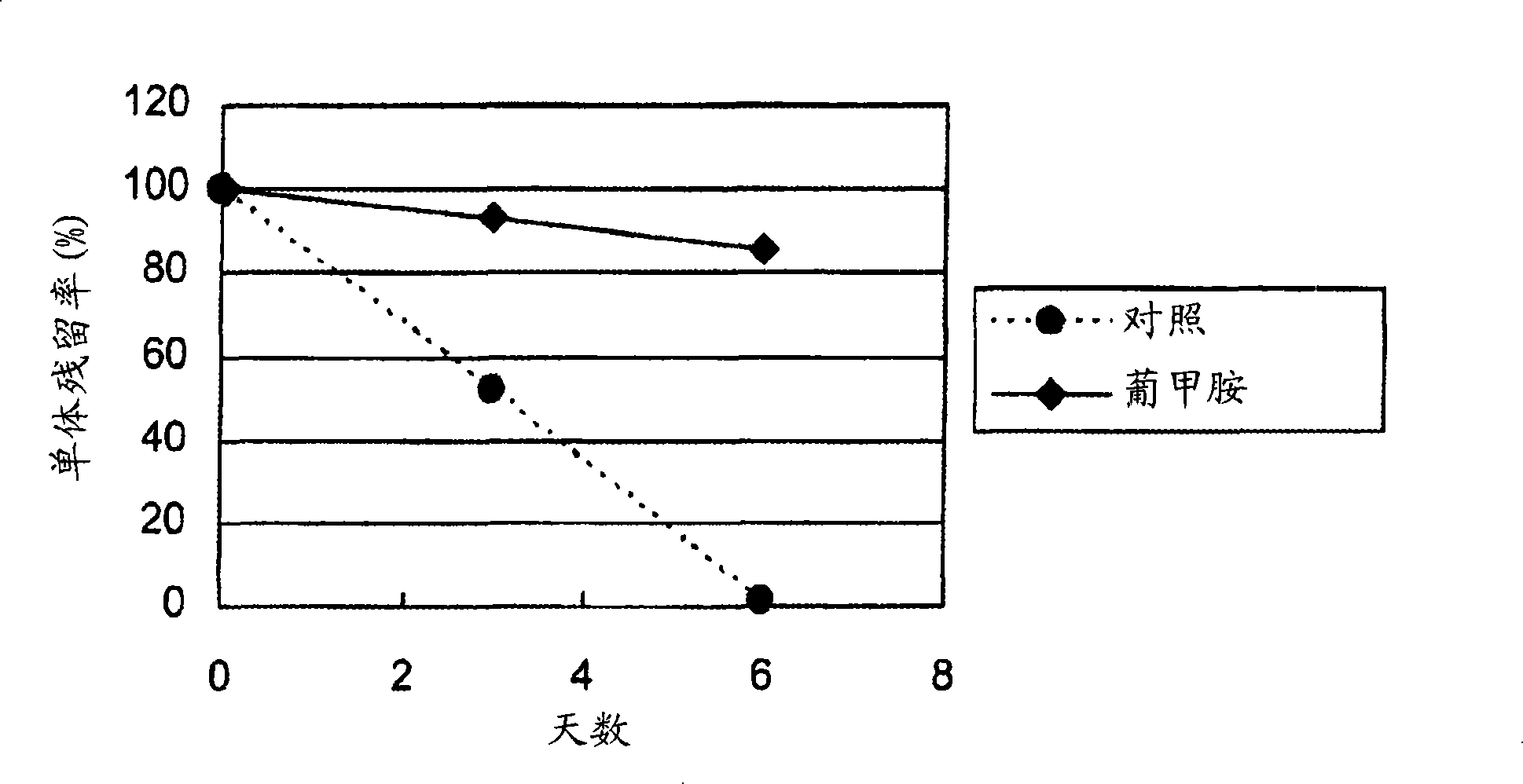
[0184] [Example 1] Research on the stabilizing effect of meglumine on hVB22B
[0185] Using highly purified sc(Fv)2, hVB22B sc(Fv)2u2-wz4 (SEQ ID NO. 1, refer to the following reference example), the effect of meglumine on inhibiting the aggregation reaction was studied. The solvent conditions and hVB22B concentration in the stability test are as follows.
[0186]
[0187] Control: 20mM sodium citrate (pH 6.5)
[0188] Meglumine: 20mM sodium citrate, pH 6.5, 10% meglumine
[0189] hVB22B u2-wz4: 1.0mg / ml
[0190] Under the above conditions, after 55°C-3 days and 6 days, use SEC (Size Exclusion Chromatography) to measure the monomer residual rate (the monomer residual rate is the monomer peak area of the thermal acceleration test sample relative to the initial state monomer % of peak area). The analysis conditions of SEC are as follows.
[0191]
[0192] Column: TSKgel Super2000sw (TOSOH)
[0193] Eluent: 50mM Sodium Phosphate, 300mM KCl, pH 7.0
[0194] Flow rate:...
Embodiment 2
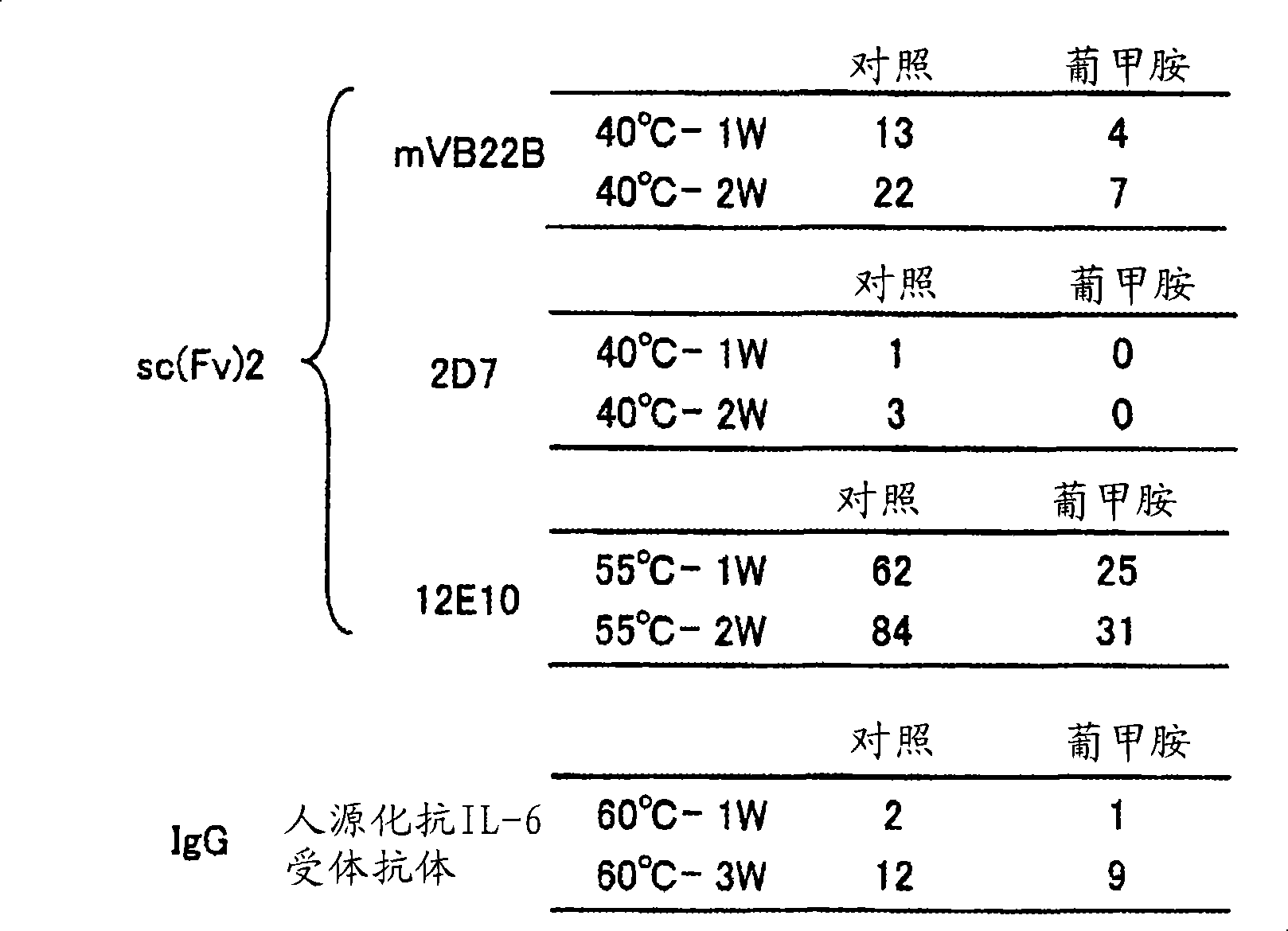
[0198] [Example 2] Meglumine to other sc (Fv) 2 molecule, and the study of the stabilizing effect of full-length antibody
[0199] sc(Fv)2 other than hVB22B shown in [Example 1]: mVB22B shown below (SEQ ID NO. 2, refer to Reference Example below), 12E10 (SEQ ID NO. 3, WO02 / 33072), 2D7 (SEQ ID NO.4, refer to Reference Example below), the stabilization effect of meglumine was studied. Also for the stabilization of humanized anti-IL-6 receptor antibody (H chain-SEQ ID NO.5, L chain-SEQ ID NO.6, WO92 / 19759) that is not sc(Fv)2 but full-length antibody IgG1 The chemical effect was studied. The solvent conditions and the concentration of each antibody in the stability test are as follows.
[0200]
[0201] Control: 20mM citrate buffer, pH 6.5
[0202] Meglumine: 20mM citrate buffer, 10% meglumine, pH 6.5
[0203] mVB22B: 28μg / ml, measured after 40°C-1 week and 2 weeks later
[0204] 2D7: 59μg / ml, measured at 40°C after 1 week and 2 weeks
[0205] 12E10: 10μg / ml, measured at ...
Embodiment 3
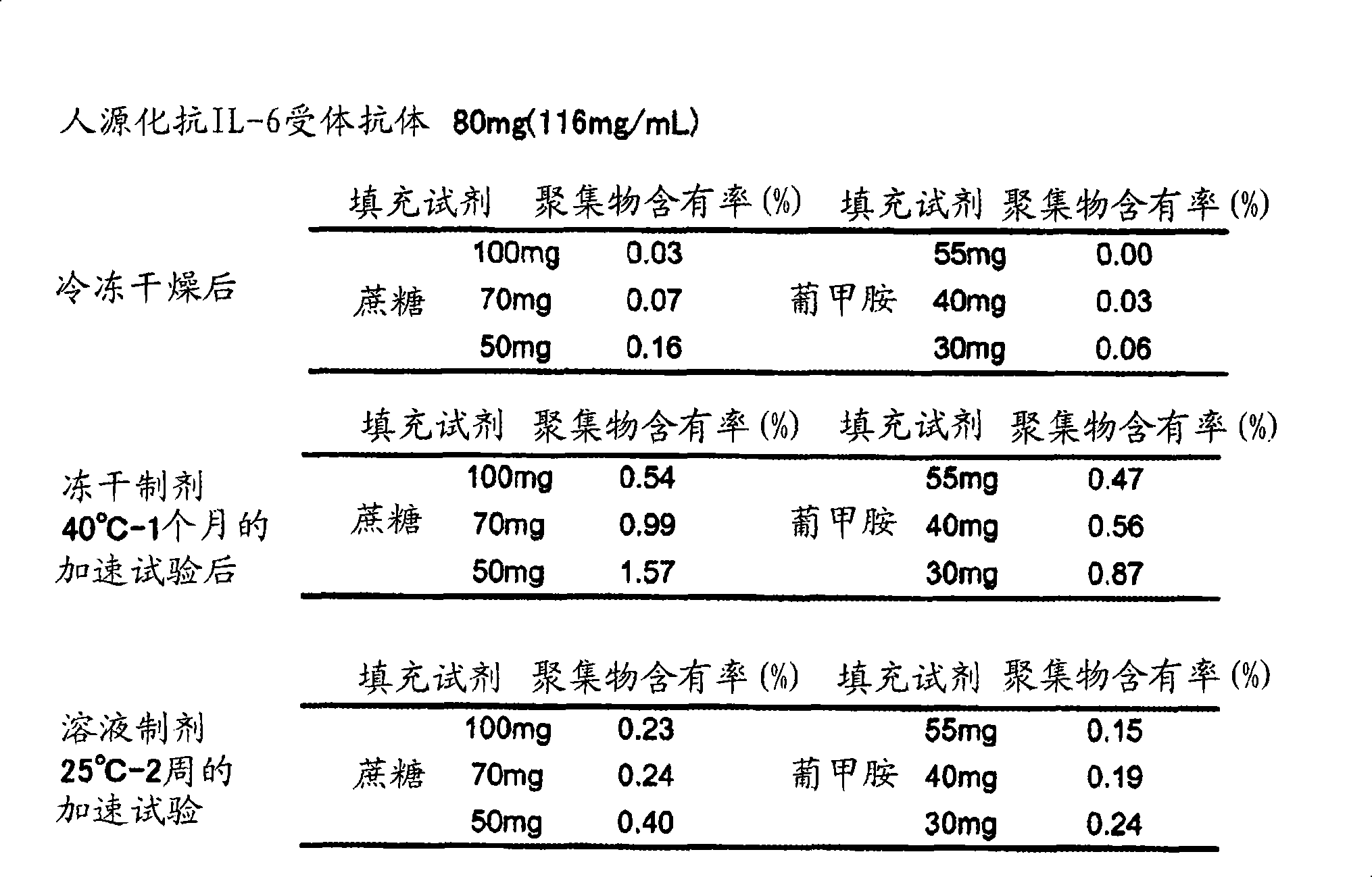
[0220] [Example 3] Research on meglumine as an IgG solution stabilizer and an excipient for freeze-dried preparations
[0221] The stabilizing effect of meglumine as a stabilizer for IgG solution preparations or as an excipient for lyophilized preparations was studied. Comparison using normal sucrose as an IgG stabilizer (excipient). Sucrose has been reported to be very effective as a lyophilized formulation of the formulation. The specimens used for the tests and the stable experimental conditions are shown below.
[0222]
[0223] Sample 1: IgG 80mg / bottle, sucrose 100mg / bottle, polysorbate 80 1mg / bottle, sodium phosphate salt 30μmol / bottle, pH 6.0
[0224] Sample 2: IgG 80mg / bottle, sucrose 70mg / bottle, polysorbate 80 1mg / bottle, sodium phosphate salt 30μmol / bottle, pH 6.0
[0225] Sample 3: IgG 80mg / bottle, sucrose 50mg / bottle, polysorbate 80 1mg / bottle, sodium phosphate salt 30μmol / bottle, pH 6.0
[0226] Sample 4: IgG 80mg / bottle, meglumine 55mg / bottle, polysorbate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com