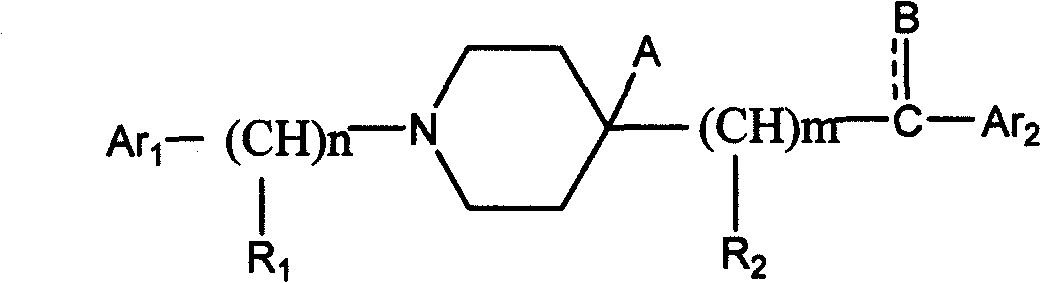
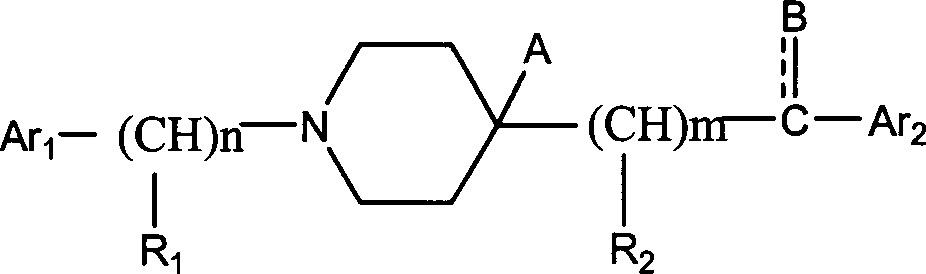
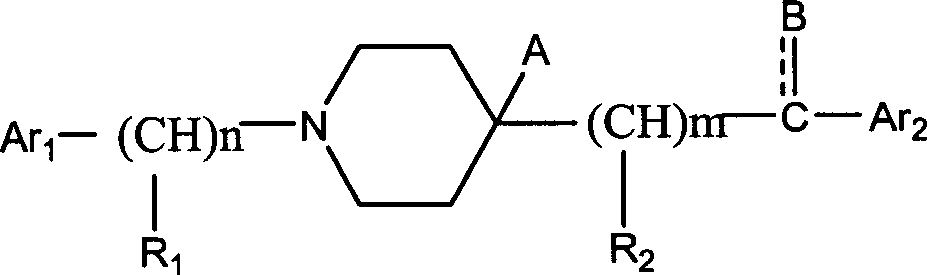
Aralkylpiperidine derivative and application thereof in preparing analgesic and sedative medicament
A technology of aralkylpiperidine and derivatives, which is applied in the field of aralkylpiperidine derivatives, and can solve problems such as reduced gastric motility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] III-1 Preparation of N-benzyl-4-phenacyl-4-piperidinol hydrochloride and N-benzyl-4-phenacyl-4-piperidinol hydrobromide:
[0159] Anhydrous cerium chloride (1.98 g, 8.0 mmol) and sodium iodide (3.6 g, 24.0 mmol) were added to 20 ml of anhydrous tetrahydrofuran solvent to form a suspension. Bromoacetophenone 1.60 (8.0mmol) and N-benzyl-4-piperidone 1.51g (8.0mmol) were dissolved in 10ml of anhydrous tetrahydrofuran, and the solution was added dropwise to the above suspension, according to the general method The operation of the synthesis and post-treatment methods of the third method gave 1.05 g of white crystals with a yield of 36%.
[0160] N-benzyl-4-phenacylmethyl-4-piperidinol was prepared by the above method, acidified with hydrobromic acid / ethanol solution to form a salt during post-treatment, and recrystallized from ethyl acetate / ethanol to obtain white crystals. Yield 32%.
[0161] Elemental analysis: (Theoretical %: C66.01, H7.20, N3.85, Cl9.74 Experimental...
Embodiment 2
[0166] III-2 N-p-chlorobenzyl-4-phenacyl-4-piperidinol hydrochloride
[0167] First prepare 4-phenacyl-4-piperidinol (II) according to the synthesis and post-treatment method of general method one, and then p-chlorobromobenzyl 1.76 (8.8mmol) and 4-phenacyl-4 - piperidinol (II) 1.75g (8.0mmol), potassium iodide 0.03g (0.2mmol) and anhydrous K 2 CO 3 3.53g (25.6mmol) was placed in anhydrous acetone (60ml), and refluxed for 8 hours. According to the post-treatment operation of General Method 2, 2.25g of white crystals were obtained with a yield of 70.5%.
[0168] Elemental analysis: (Theoretical %: C60.31, H6.33, N3.52, Cl17.80 Experimental %: C60.42, H6.15, N3.30, Cl17.96)
[0169] 1 HNMR (DMSO-d 6 ): 1.80-2.10(m, 4H, piperidine-H), 3.00-3.20(m, 4H, piperidine-H), 3.15(s, 2H, CH 2 CO), 4.25-4.40 (m, 2H, PhCH 2 ), 4.98 (s, IH, piperidine-NHCl), 7.20-8.10 (m, 9H, ArH), 10.5-11.5 (2B, 1H, piperidine-OH).
[0170] MS: m / z 344 (M + )
Embodiment 3
[0172] III-3 N-p-fluorobenzyl-4-phenacyl-4-piperidinol hydrochloride
[0173] First prepare 4-phenacyl-4-piperidinol (II) according to the synthesis and post-treatment method of general method one, and then p-fluorobromobenzyl 1.66 (8.8mmol) and 4-phenacyl-4 - piperidinol (II) 1.75g (8.0mmol), potassium iodide 0.03g (0.2mmol) and anhydrous K 2 CO 3 3.53g (25.6mmol) was placed in anhydrous acetone (60ml), and refluxed for 8 hours. According to the post-treatment operation of General Method 2, 2.06g of white crystals were obtained with a yield of 67.5%.
[0174] Elemental analysis: (Theoretical %: C62.90, H6.60, N3.67, Cl9.28 Experimental %: C60.87, H6.45, N3.39, Cl9.56)
[0175] 1 HNMR (DMSO-d 6 ): 1.80-2.10(m, 4H, piperidine-H), 3.00-3.20(m, 4H, piperidine-H), 3.13(s, 2H, CH 2 CO), 4.25-4.40 (m, 2H, PhCH 2 ), 5.02 (s, IH, piperidine-NHCl), 7.20-8.10 (m, 9H, ArH), 10.6-11.2 (2B, 1H, piperidine-OH).
[0176] MS: m / z 328 (M + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com