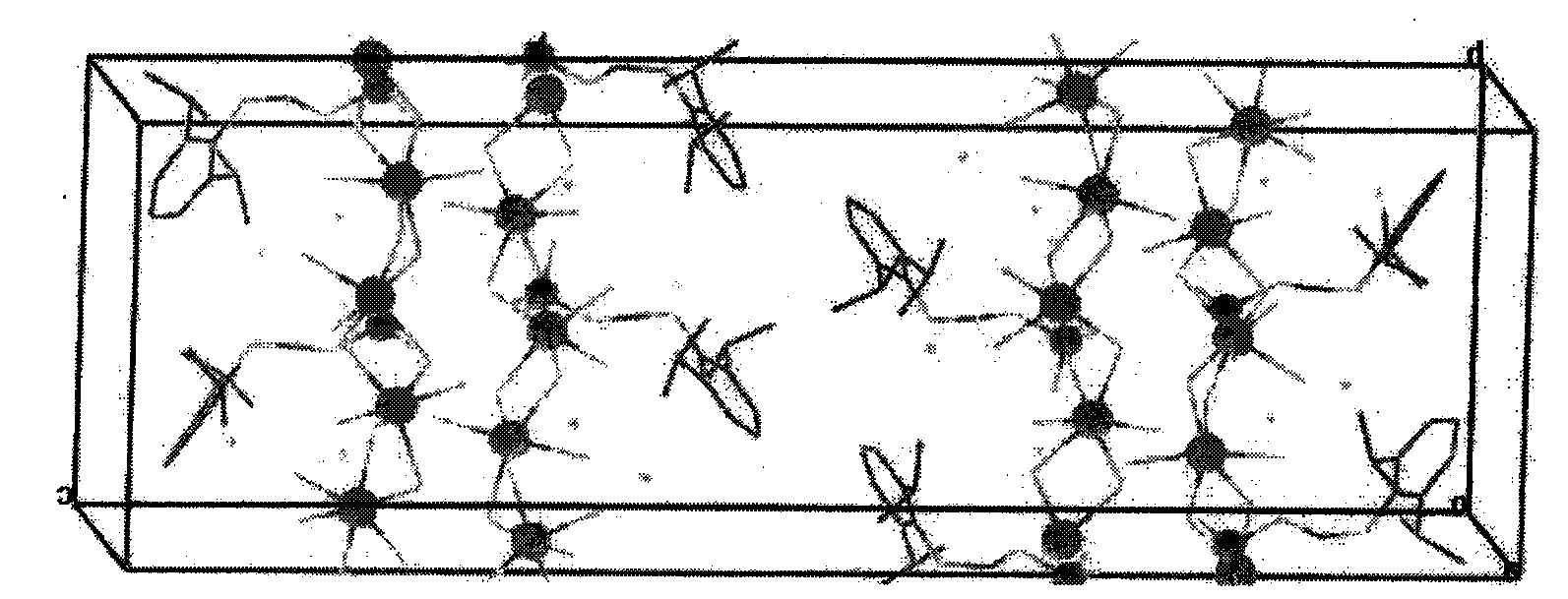
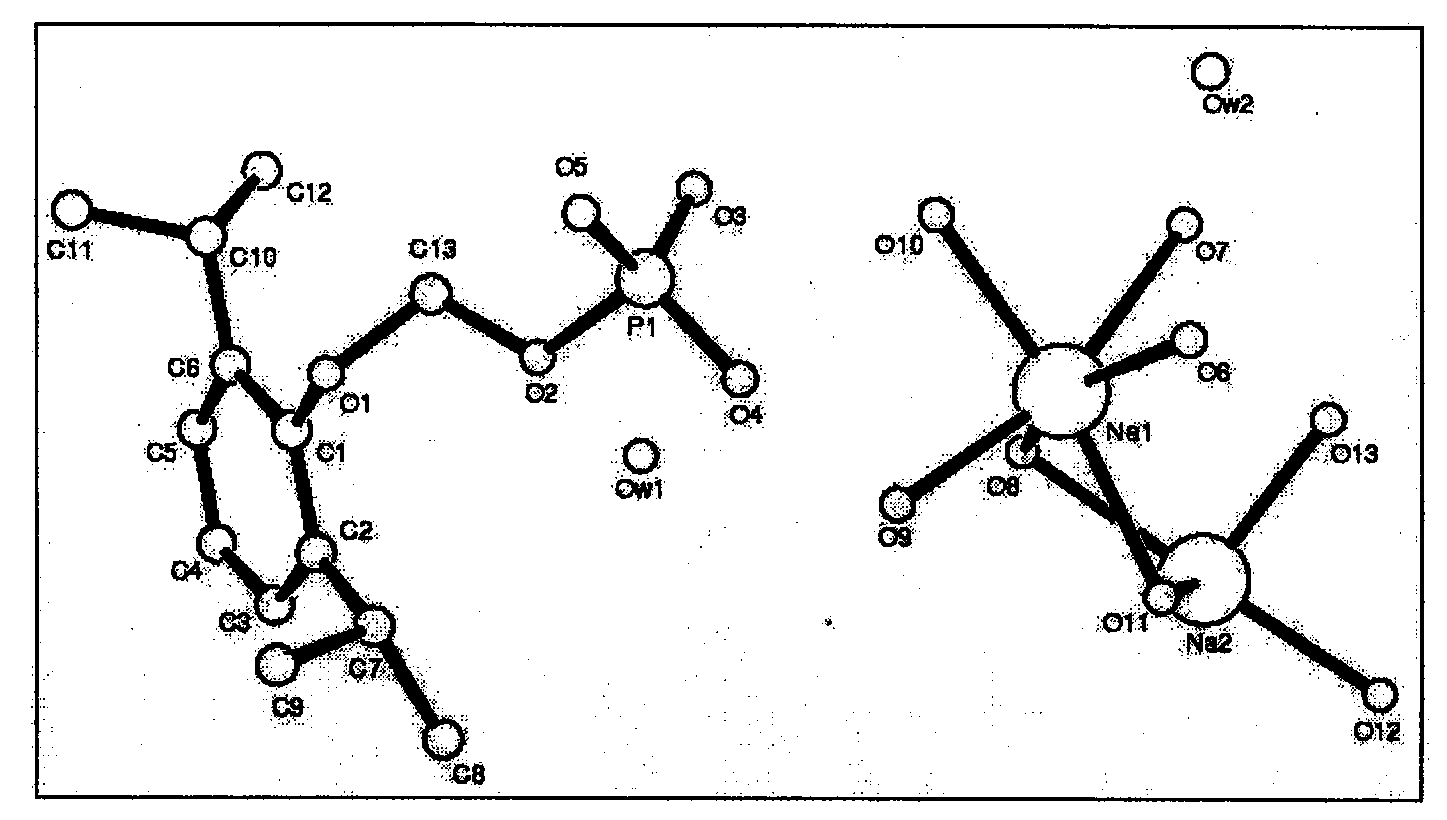
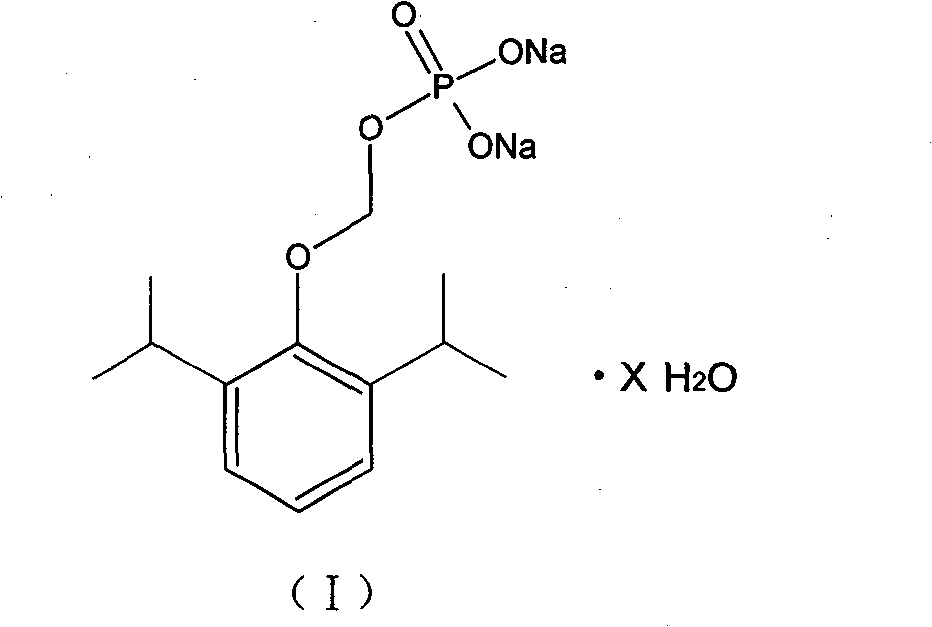
Substituted phenol for methylal phosphate anesthetic and sedative drugs and preparation method thereof
A technology of methylal phosphate disodium salt and acetal phosphate, which is applied in the field of phenol-substituted methylal phosphate anesthesia and sedative medicinal compounds and can solve the problems of difficult control of water content, strong hygroscopicity, and quality problems. Standard Difficulties and Other Issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 13.0g of propofol phosphate disodium salt (commercially purchased or prepared according to the current literature method) into a 50ml single-mouth bottle, add 40ml of water and stir to dissolve at 50°C, add an appropriate amount of activated carbon to decolorize for 15 minutes after complete dissolution, filter, and dissolve 400ml of acetone Add it to the filtrate under stirring, stir to room temperature and place it under refrigeration until the crystallization is complete. Filtrate, wash with a small amount of acetone, and dry to obtain 16.1 g of white crystals of propofol phosphate disodium salt hydrate. After drying at 105°C to constant weight, the water content was 35%, and the yield was 80.5%. The infrared absorption spectrum was measured by the potassium bromide tablet method (instrument: American NICOLET MX-1 FT-IR infrared spectrometer), and the IR analysis and detection results are shown in Table 1.
[0023] Table 1 IR measurement data and analysis results...
Embodiment 2
[0027] Add 13.0g of propofol phosphate disodium salt of the formula (III) into a 50ml one-mouth bottle, add 26ml of water and stir to dissolve at 50°C, add an appropriate amount of activated carbon to decolorize for 15 minutes after complete dissolution, filter, and add the filtrate to 300ml iso In propanol, heat to reflux, stir to room temperature, place in the freezer and refrigerate until the crystallization is complete. Filtrate, wash, and dry to obtain 13.2 g of white crystals of propofol phosphate disodium salt hydrate, which was dried at 105° C. to constant weight. The measured water content was 21%, and the yield was 80.2%. The results of IR analysis are shown in Table 2.
[0028] Table 2 IR measurement data and analysis results
[0029]
Embodiment 3
[0031] Add 13.0 g of propofol phosphate disodium salt of formula (III) (commercially purchased or prepared according to the current literature method) into a 50 ml single-mouth bottle, add 30 ml of water and stir to dissolve at 50 ° C, after complete dissolution, add an appropriate amount of activated carbon to decolorize for 15 minutes, Filtrate, add the filtrate to 400ml of N,N-dimethylformamide under stirring, heat to reflux, stir to room temperature and place in a freezer to refrigerate until the crystallization is complete. Filtrate, wash, and dry to obtain 12.3 g of white crystals of propofol phosphate disodium salt hydrate, which was dried at 105° C. to constant weight. The measured water content was 10%, and the yield was 85.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com