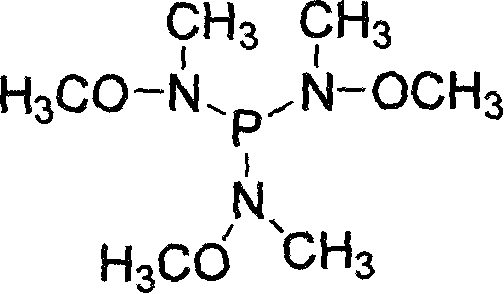
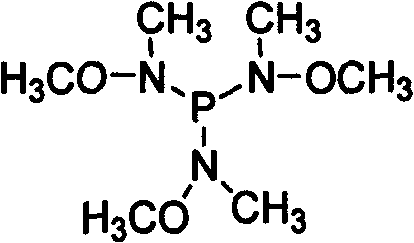
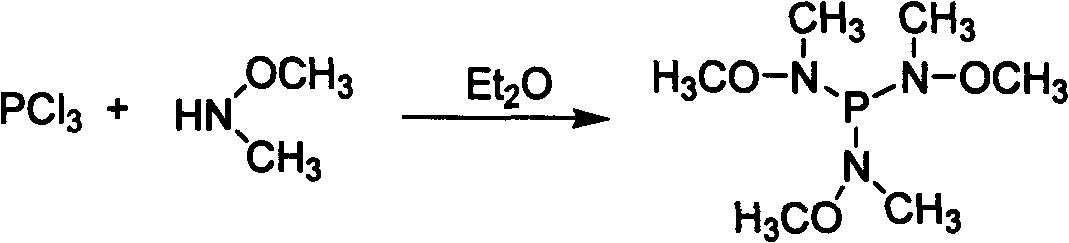
Preparation of P(NMeOMe)3 agent and application thereof in synthesizing N-methyl-N-methoxylamide
A technology of methoxyamide and trimethoxyphosphonamidite, which is used in the synthesis of N-methyl-N-methoxyamide intermediates and organic synthesis intermediates. The reagent is used in the synthesis of N-methyl-N- The application field in methoxyamide can solve the problems of removal, waste of raw materials, yield, expensive deoxyfluorinating agent, etc., and achieve the effect of easy post-processing, high synthesis efficiency and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the synthesis of N-methyl-N-methoxybenzamide
[0024] Step 1, P(NOMeMe) 3 Synthesis: under ice-salt bath cooling and nitrogen protection, in a 250ml three-necked flask, add Me(MeO)NH 62mmol (3.78g) and NEt 3 70mmol (20ml), in 100ml anhydrous ether mixture, slowly add PCl dropwise with stirring 3 20 mmol (2.74 g) of anhydrous ether mixture was added dropwise, and allowed to warm to room temperature naturally. The mixture was stirred overnight, heated for 4 hours the next day, lowered to room temperature and filtered to remove the ammonia salt, and the solvent was distilled off to obtain a pale yellow liquid. Under reduced pressure distillation, 2.81 g of a colorless liquid was obtained, which was the target product 3. Yield 66.7%. Its reaction formula is as follows:
[0025]
[0026] Step 2. Synthesis of N-methyl-N-methoxyamide: In a 50mL three-necked flask, compound P(NOMeMe) 3 0.211 g (1 mmol) was dissolved in 10 ml of toluene, 0.244 g (2 mmol...
Embodiment 2
[0032]Example 2. Synthesis of N-methyl-N-methoxydithiophene-2-carboxamide
[0033] Step 1. P(NOMeMe) 3 Synthesis of : the same as in Example 1.
[0034] Step 2. Synthesis of N-methyl-N-methoxyamide: In a 50mL three-necked flask, compound P(NOMeMe) 3 0.211 g (1 mmol) was dissolved in 10 ml of toluene, 0.368 g (2 mmol) of dithiophene-2-carboxylic acid was added, and the mixture was heated at 60°C for 30 minutes under a nitrogen atmosphere. After the reaction of the raw materials was detected by TLC, it was cooled to room temperature, add saturated NaHCO 3 An aqueous solution quenched the reaction. Extract with ether, MgSO 4 dry. The compound N-methyl-N-methoxydithiophene-2-carboxamide was obtained by column chromatography. The yield was 96.6%. Its reaction formula is as follows:
[0035]
[0036] Step 3. Detection of N-methyl-N-methoxy amide compounds: The N-methyl-N-methoxy amide compounds synthesized above were subjected to 1 HNMR, IR, 13 CNMR detection, the produ...
Embodiment 3
[0040] Example 3. Synthesis of N-methyl-N-methoxy-2-(N-p-methylbenzenesulfonyl) phenylacetamide
[0041] Step 1. P(NOMeMe) 3 Synthesis: same as Example 1;
[0042] Step 2. Synthesis of N-methyl-N-methoxyamide: In a 50mL three-necked flask, compound P(NOMeMe) 3 0.211 g (1 mmol) was dissolved in 10 ml of toluene, 0.67 g (2 mmol) of p-toluenesulfonic acid-protected aniline was added, and the mixture was heated at 60° C. for 30 minutes under a nitrogen atmosphere. After TLC detection, the reaction of the raw materials was completed. After cooling to room temperature, saturated NaHCO was added. 3 An aqueous solution quenched the reaction. Extract with ether, MgSO 4 dry. The target compound, N-methyl-N-methoxy-2-(N-p-methylbenzenesulfonyl)phenylacetamide, was obtained by column chromatography. The yield was 94.7%. Its reaction formula is as follows:
[0043]
[0044] Step 3. Detection of N-methyl-N-methoxy amide compounds: The N-methyl-N-methoxy amide compounds synthesiz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com