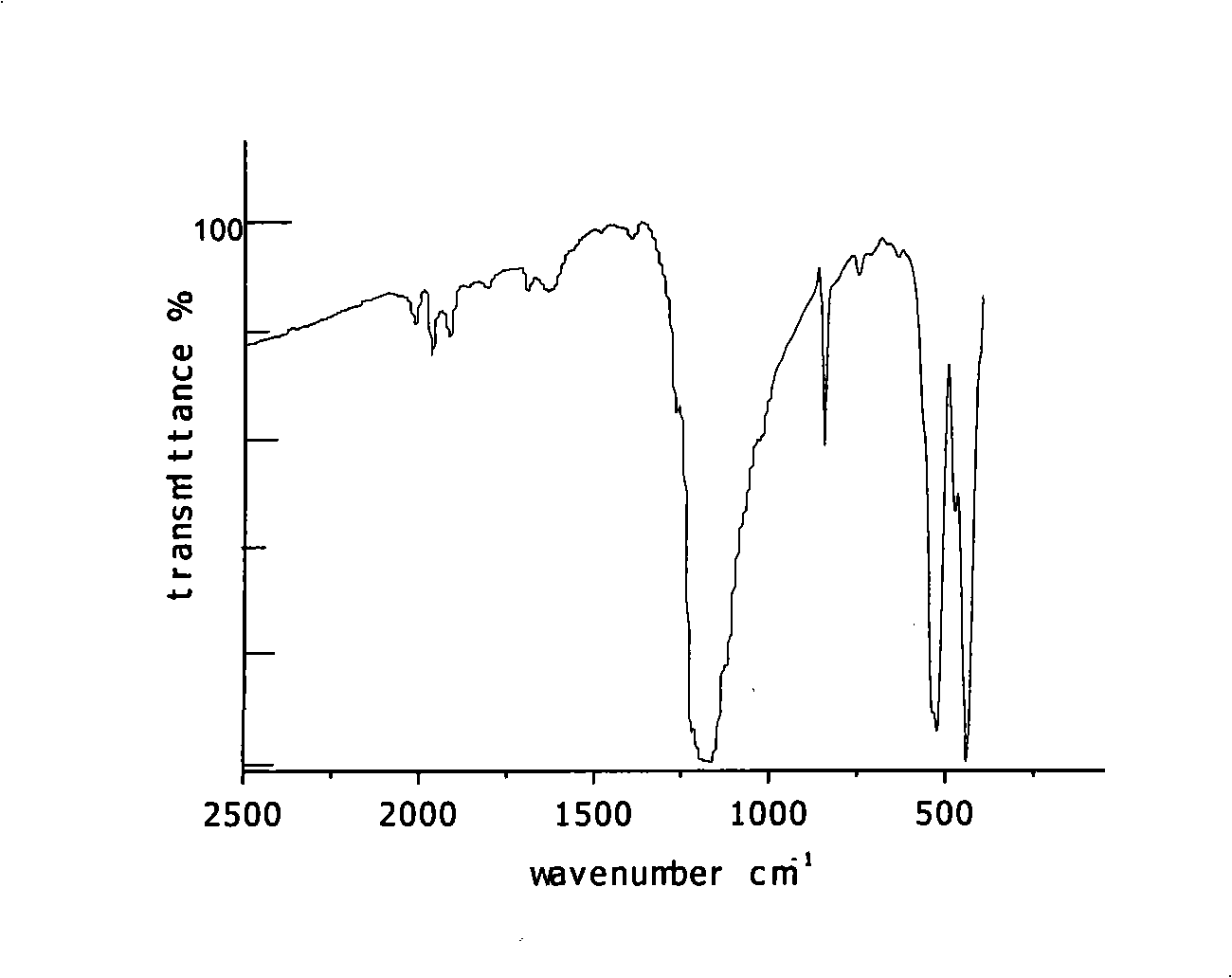
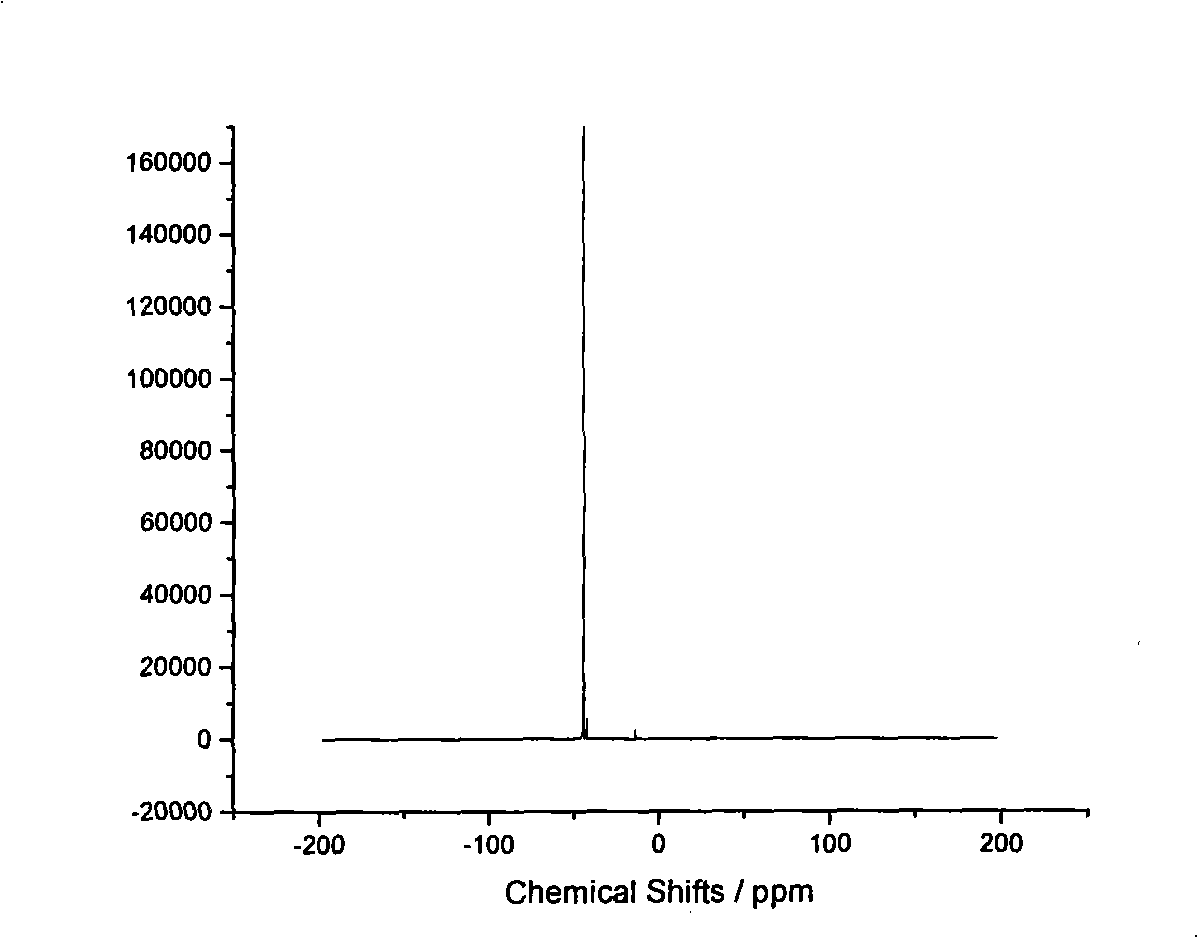
Technique for preparing hexabromocyclophosphazene trimer
A technology of hexabromocyclotripolyphosphazene and a new process is applied in the field of chemical synthesis, can solve the problems of low cost, high cost, low yield and the like, and achieves the effects of mild reaction process conditions, high success rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 15g dry ammonium bromide, 10ml phosphorus tribromide, 200ml chlorobenzene and 6.0ml, 0.5g CaCl 2 , a composite catalyst composed of 0.5g ZnO. Heating to reflux with stirring to boiling, quickly drop 3.0ml of liquid Br into the three-necked flask 2 , Control the temperature of the reaction system at 130°C to 140°C. After reacting for 1 hour, add 1.0 g of dry ammonium bromide and continue to slowly add 3.0 ml of liquid Br 2 . After 1 hour, 1.0 g of dry ammonium bromide was added, and the reaction was continued for 4 hours before heating was stopped. The unreacted ammonium bromide was filtered out while hot, and the filtrate was distilled under reduced pressure until a small amount of crystals were precipitated. The distillation residue was rapidly cooled, and white crystals precipitated. The obtained white crystals were dissolved in toluene and recrystallized to obtain a white crystalline hexabromocyclotrimeric phosphazene product with a yield of 51%.
Embodiment 2
[0022] Add 15g of dry ammonium bromide, 10ml of phosphorus tribromide, 200ml of chlorobenzene, and 6.0ml of pyridine, 0.5g of CuCl 2 and 0.5g CaO composite catalyst. Heating to reflux with stirring to boiling, quickly drop 3.0ml of liquid Br into the three-necked flask 2 , Control the temperature of the reaction system at 130°C to 140°C. After reacting for 1 hour, then add 1.0 g of dry ammonium bromide, continue to slowly add 3.0 ml of liquid Br2. After 1 hour, add 1.0 g of dry ammonium bromide, and continue the reaction for 4 hours before stopping heating. The unreacted ammonium bromide was filtered out while hot, and the filtrate was distilled under reduced pressure until a small amount of crystals were precipitated. The distillation residue was rapidly cooled, and white crystals precipitated. The obtained white crystals were dissolved in toluene and recrystallized to obtain a white crystalline hexabromocyclotrimeric phosphazene product with a yield of 43%.
Embodiment 3
[0024] Add 15g dry ammonium bromide, 10ml phosphorus tribromide, 200ml chlorobenzene and 6.0ml 4-picoline, 0.5g CoCl 2 , 0.5g NiCl 2 composed of composite catalysts. Heating to reflux with stirring to boiling, quickly drop 3.0ml of liquid Br into the three-necked flask 2 , Control the temperature of the reaction system at 130°C to 140°C. After reacting for 1 hour, add 1.0 g of dry ammonium bromide and continue to slowly add 3.0 ml of liquid Br 2 . After 1 hour, 1.0 g of dry ammonium bromide was added, and the reaction was continued for 4 hours before heating was stopped. The unreacted ammonium bromide was filtered out while hot, and the filtrate was distilled under reduced pressure until a small amount of crystals were precipitated. The distillation residue was rapidly cooled, and white crystals precipitated. The resulting white crystals were dissolved in toluene and recrystallized to obtain a white crystalline hexabromocyclotrimeric phosphazene product with a yield of 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com