Emulsion composition comprising prostaglandin E1
A composition, the technology of polyoxyethylene sorbitan ester, is applied in the field of emulsified composition, and can solve the problems such as inability to provide enhancement, increase cycle time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Including PGE 1 And preparation of the emulsified composition TLC01 of Polysorbate 80 (Polysorbate 80)
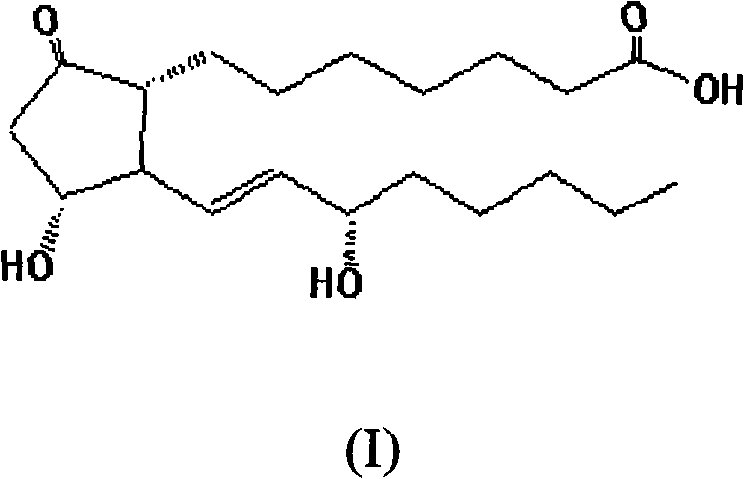
[0038] Prepare PGE including formula (I) in this example 1 And a non-proton-donating surfactant, an emulsified composition of polyoxyethylene sorbitan 80. A method similar to this embodiment can also be used to prepare any other PGE 1 And the emulsified composition of non-proton-donating surfactant.
[0039] PGE of formula (I) 1 Other chemicals were purchased from Sigma (USA). Polyoxyethylene sorbitan ester 80 was purchased from Sigma Company (USA), NOF Company (Japan) or Imperial Chemical Industries PLC (London, UK). MCT oil (a mixture of triglycerides, the fatty acids in the glyceride structure mainly contain not less than about 95% caprylic acid and capric acid), such as LIPOID purchased from Lipoid GmbH (Germany) Or Panacet purchased from NOF (Japan) . Lecithin acylcholine (EPC) (purity: 95-100% and purity: 80-85%) was purchased from Avanti (USA), NOF (Japan), or L...
Embodiment 2
[0042] Including PGE 1 And preparation of emulsified composition of non-proton-donating surfactant
[0043] Different emulsified compositions were prepared using a method similar to that described in Example 1. These emulsified compositions include PGE 1 And different non-proton-donating surfactants.
[0044] Non-proton-donating surfactants are obtained from various sources. Polyethylene glycol-15-hydroxystearate ( HS15) and Polyoxyl 35 castor oil ( EL) was obtained from BASF, Germany. D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) was obtained from Eastman Company, USA. 3-(N,N-Dimethylpalmitylammonium)propanesulfonate and dodecyltrimethylammonium bromide were obtained from Sigma, USA. DSPE-mPEG 2000 was purchased from Avanti Company in the United States or NOF Company in Japan.
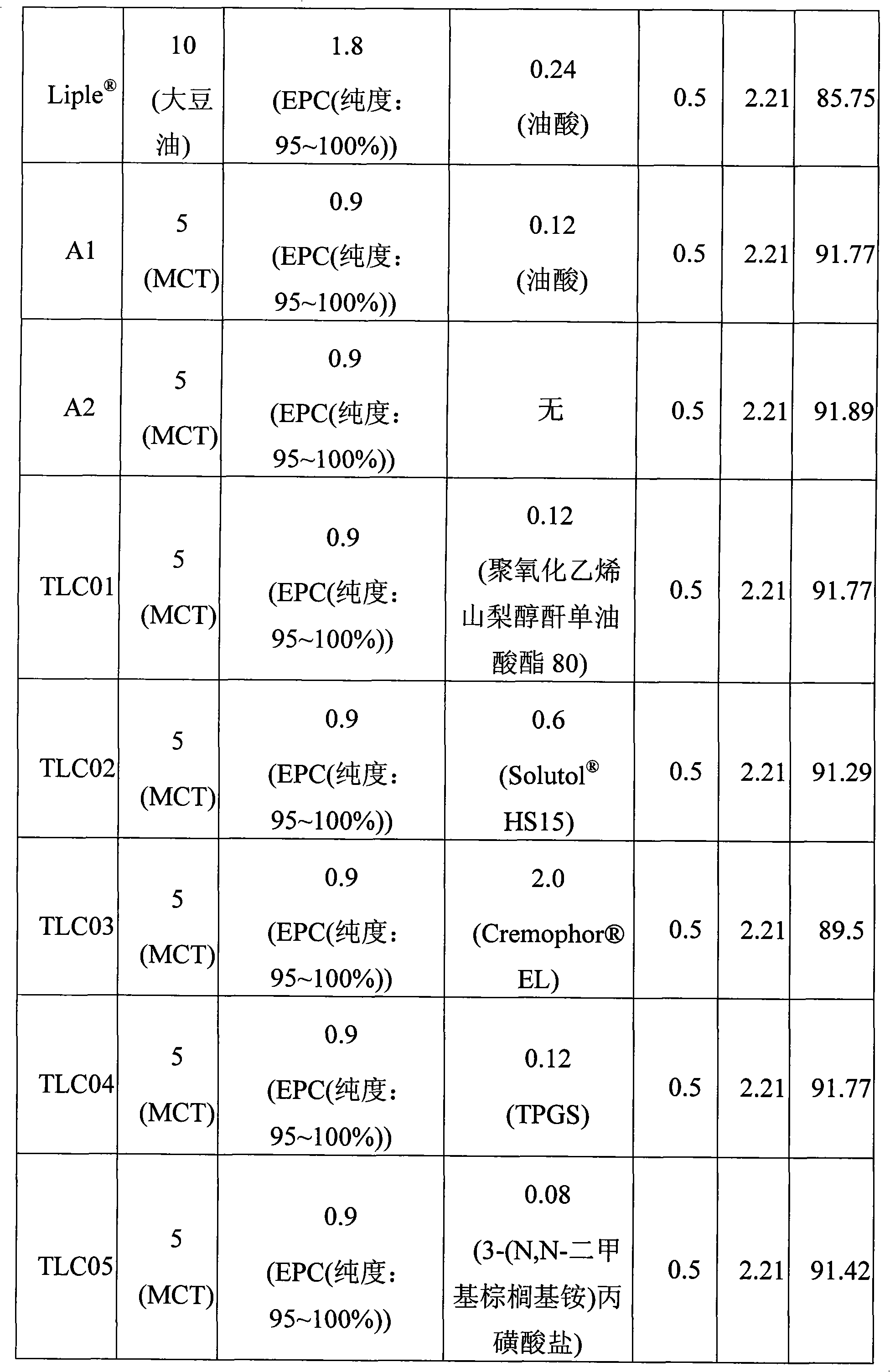
[0045] The ingredients and amounts of various emulsified compositions are listed in Table I.
[0046] Table I Composition and amount of emulsified composition
[0047]
[0048]
[0049]...
Embodiment 3
[0051] PGE in emulsified composition 1 Determination of storage stability
[0052] In this example, the PGE in the array of emulsion composition was measured 1 The storage stability. Use a method similar to this example to determine the inclusion of PGE 1 Any PGE in any emulsion composition of 1 The storage stability.
[0053] Use commercially available products (Mitsubishi Pharmaceutical Co., Ltd. (Tokyo, Japan)) as a control group for comparison with emulsified compositions according to specific examples of the present invention. The ingredients and amounts of various emulsified compositions are listed in Table I for comparison.
[0054] PGE in an emulsified composition stored in a sealed transparent ampoule and placed in a dark place 1 The stability of the emulsified composition is the residual PGE measured by HPLC analysis after storage at 40°C for one week or one month 1 Expressed as a percentage. PGE in the emulsified composition 1 The stability of the product can also be o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com