Stable lornoxicam solution agent, preparation method and application thereof
A technology of lornoxicam and solution, which is applied in the field of meglumine and phosphate solution and salt, and can solve problems such as turbidity and unstable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of lornoxicam injection
[0040] Lornoxicam 4g
[0041] Mannitol 50g
[0042] Meglumine 1g
[0043] Disodium hydrogen phosphate 0.6g
[0044] 2% sodium hydroxide solution appropriate amount
[0045]
[0046] Dilute to 1000ml with water for injection
[0047] Pack according to the specifications required for clinical treatment (2ml / branch, 5ml / branch or 10ml / branch).
Embodiment 2
[0048] Example 2 Preparation of lornoxicam injection
[0049] Lornoxicam 4g
[0050] Mannitol 50g
[0051] Meglumine 4g
[0052] Disodium hydrogen phosphate 2.4g
[0053] 2% sodium hydroxide solution appropriate amount
[0054]
[0055] Dilute to 1000ml with water for injection
[0056] Pack according to the specifications required for clinical treatment (2ml / branch, 5ml / branch or 10ml / branch).
Embodiment 3
[0057] Example 3 Preparation of lornoxicam freeze-dried preparation
[0058] Lornoxicam 4g
[0059] Mannitol 50g
[0060] Meglumine 1g
[0061] Trisodium hydrogen phosphate 0.6g
[0062] 2% sodium hydroxide solution appropriate amount
[0063]
[0064] Dilute to 1000ml with water for injection
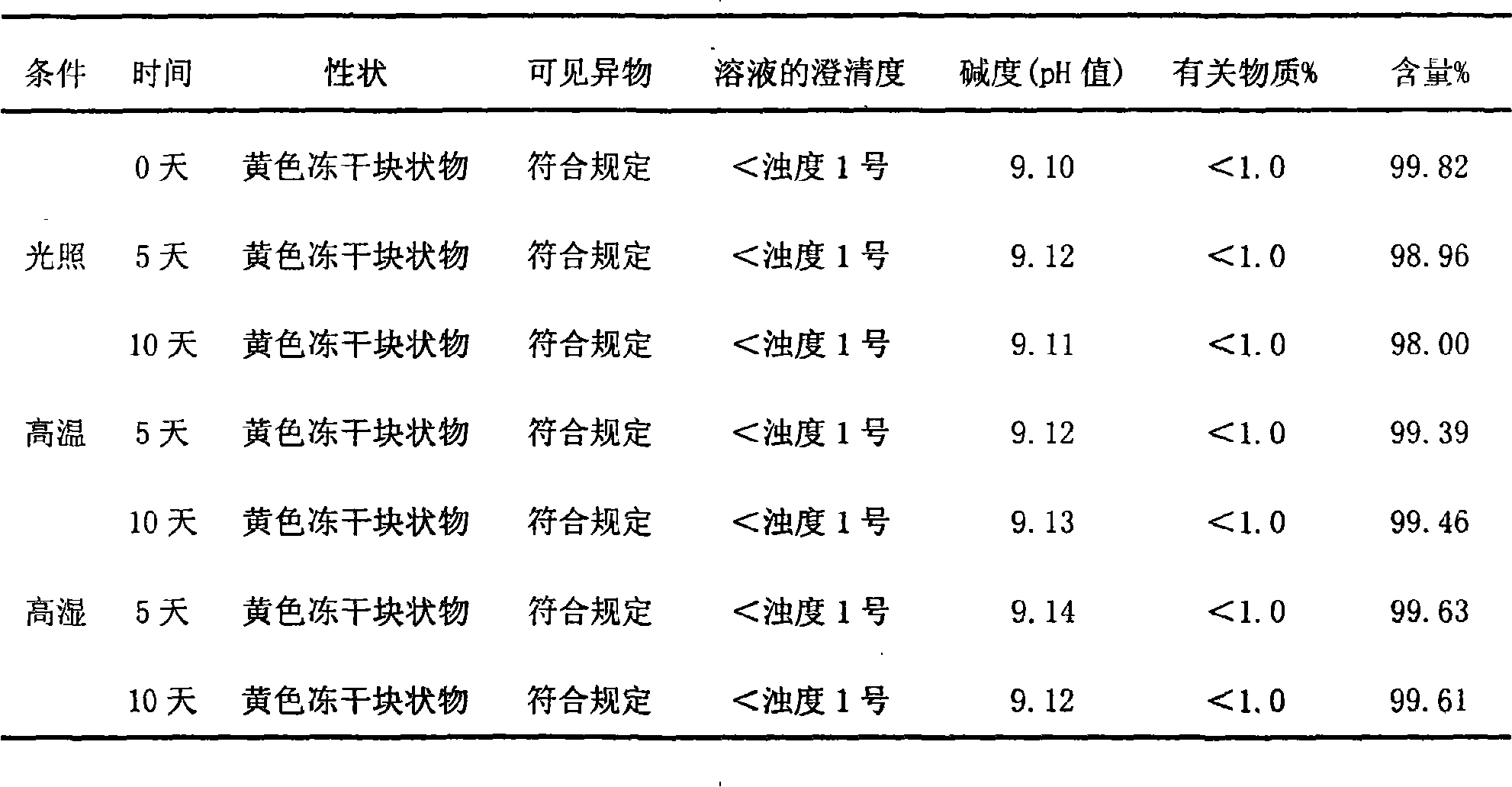
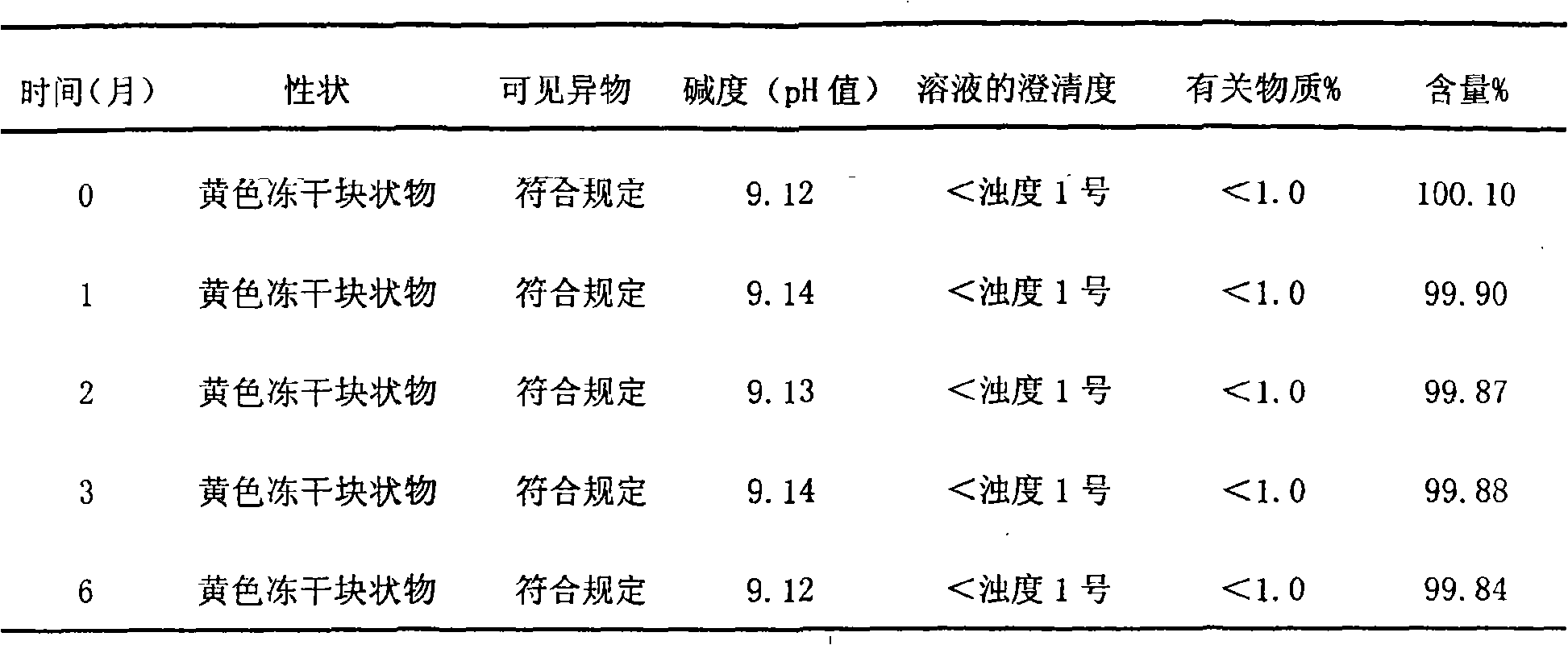
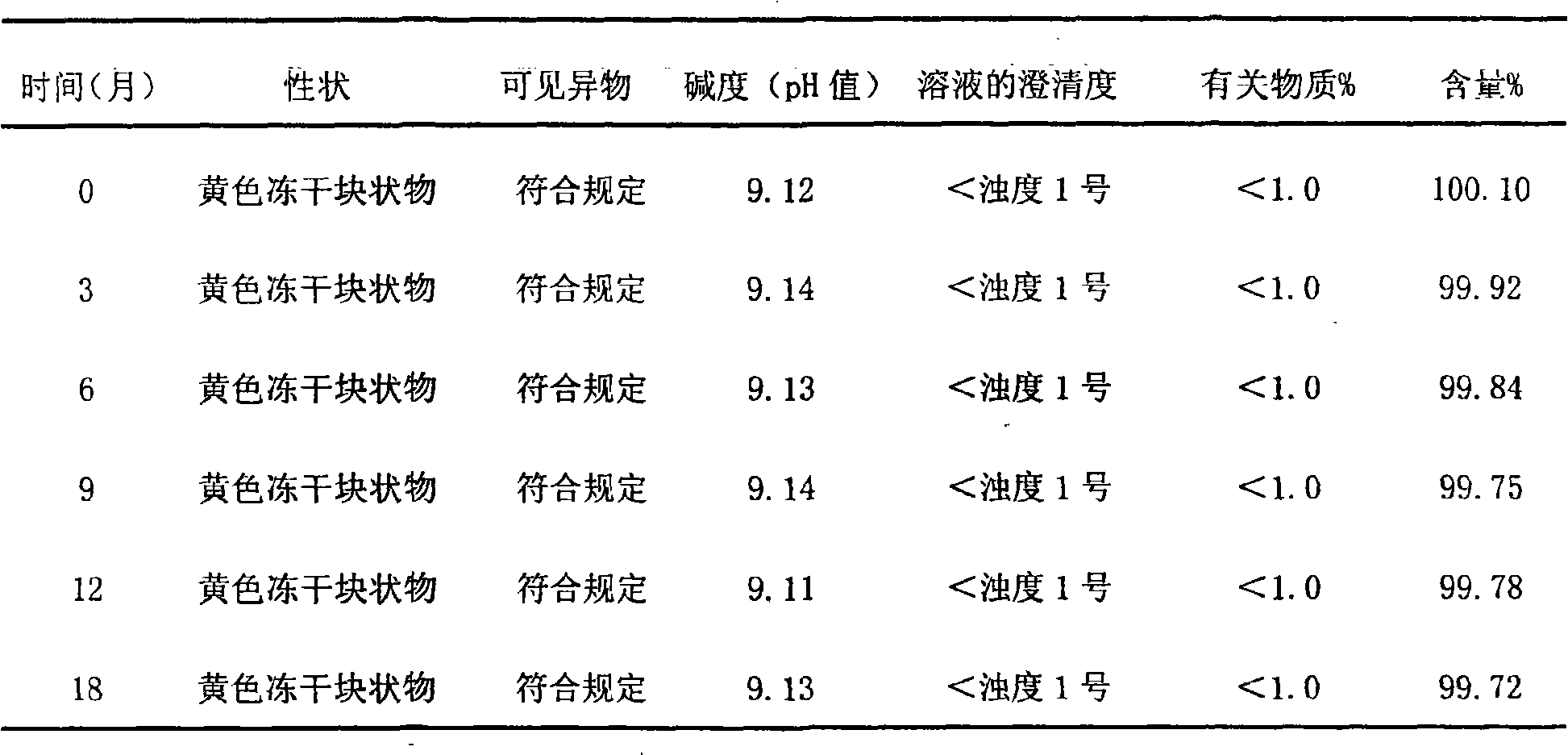
[0065] Gained injection is subpackaged into 2ml / branches under aseptic conditions, and freeze-dried to obtain its freeze-dried preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com