Method for detecting identical antigen expression on identical sample
A technique for specimens and antigens, applied in the field of immunopathology detection, can solve the problems of inconsistent experimental conditions, labor-intensive, material resources, and biased results, and achieve the effect of less harsh experimental conditions, low background, and consistent conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
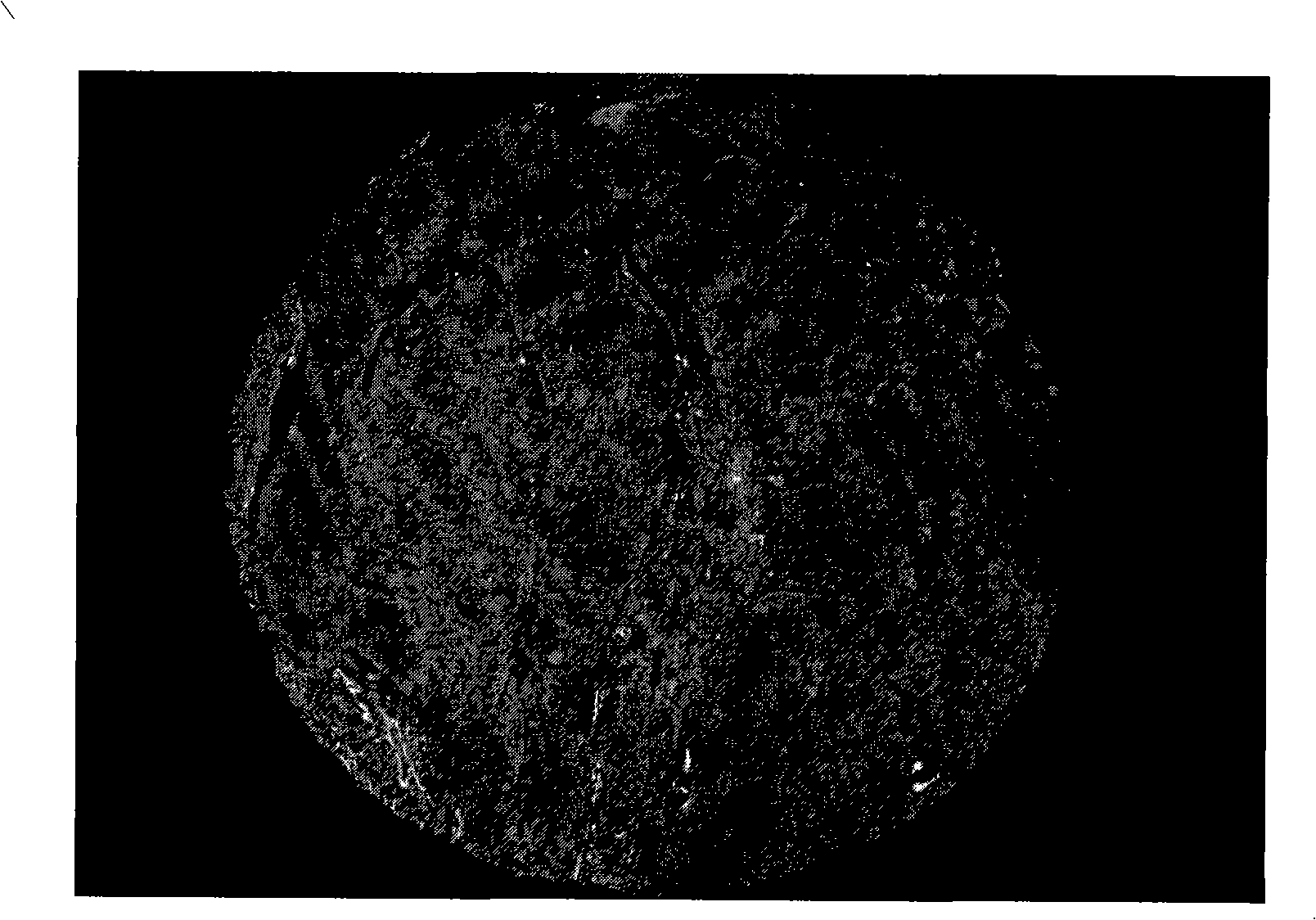
[0030] Example 1 detects the expression of Caveolin-1 protein on a lung cancer tissue chip, and the steps are:
[0031] 1.4 μm thick lung cancer tissue chips were dewaxed, hydrated, and washed with TBS (pH7.4) for 2 to 4 times, each time for 3 minutes;
[0032] 2. 0.01M pH6.0 citrate buffer, microwave antigen retrieval, naturally cool to room temperature (20-25°C, the same below), wash with TBS 2-4 times, 3min each time;
[0033] 3.2% BSA (bovine serum albumin) blocking buffer (that is, 2g BSA dissolved in 100ml TBS solution) and incubated in a wet box at 37°C for 30-35min;
[0034] 4. Add Caveolin-1 (1:100) antibody dropwise, incubate in a wet box at 37°C for 1.5-2 hours or in a refrigerator at 4°C overnight, rinse with TBS-T (pH7.4) for 3min / time x (2-4) times;
[0035] Incubate with 5.2% BSA blocking buffer in a wet box at 37°C for 10-15 minutes;
[0036] 6. Add biotinylated goat anti-rabbit IgG dropwise, incubate in a wet box at 37°C for 30-35 minutes, rinse with TBS-T f...
example 2
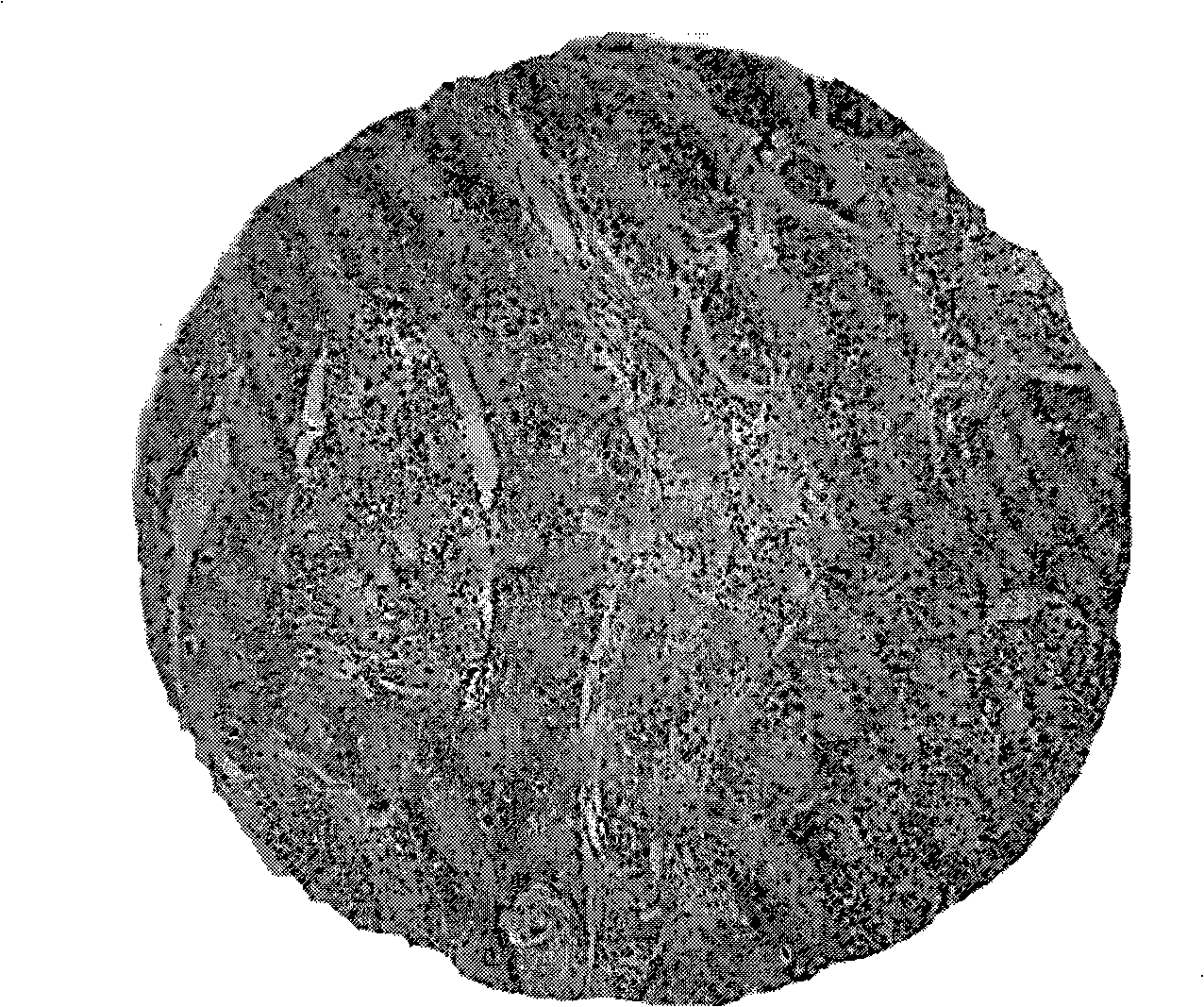
[0045] Example 2 detects the expression of Caveolin-1 protein on lung cancer cell line A549 cells, and the steps are:
[0046] 1. After A549 cells are fixed, add TBS (pH7.4) to wash 2-4 times, each time for 3 minutes, then add permeabilization solution (0.1% Triton-X 100) and incubate at room temperature for 5-10 minutes, and finally wash with TBS 2-4 times , 3 minutes each time;
[0047] Incubate with 2.2% BSA blocking buffer in a humid box at 37°C for 30-35 minutes;
[0048] 3. Add Caveolin-1 (1:100) antibody dropwise, incubate at 37°C for 1.5-2 hours or overnight in a refrigerator at 4°C, rinse with TBS-T (pH7.4) for 3min / time x (2-4) times;
[0049] Incubate with 4.2% BSA blocking buffer in a wet box at 37°C for 10-15 minutes;
[0050] 5. Add biotinylated goat anti-rabbit IgG (50-200μl) dropwise, incubate in a wet box at 37°C for 40-45min, rinse with TBS-T for 3min / time x (2-4) times;
[0051] Incubate with 6.2% BSA blocking buffer in a humid box at 37°C for 10-20 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com