Preparation of Dandengtongnao medicinal formulation and quality control method
A quality control method and a technology of pharmaceutical preparations, applied in the direction of drug combination, drug delivery, and pharmaceutical formulations, can solve problems such as incomplete quality control methods and unreasonable extraction and preparation processes, so as to monitor drug quality, shorten production cycle, and ensure The effect of the treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
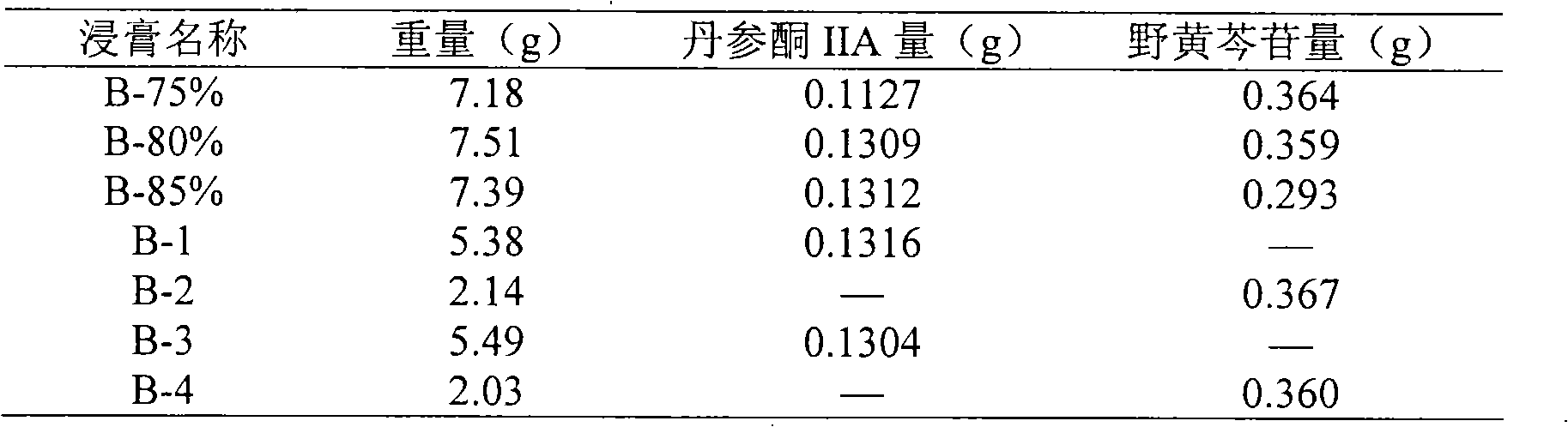
[0078]Ligusticum chuanxiong 555g, extract the volatile oil by steam distillation for later use, or wrap the extracted volatile oil with β-cyclodextrin with 3 times the weight of the volatile oil to form an inclusion complex, dry and pulverize to obtain the volatile oil inclusion complex powder; then extract the residue after extracting the volatile oil Merge with 835g of Pueraria lobata root, add water and decoct 3 times, 1 hour for the first time, 0.5 hours for the second and third times, the amount of water used is 10, 8, and 6 times the total weight of Rhizoma Chuanxiong and Pueraria lobata root respectively, combine the decoction, filter, and concentrate the filtrate to The relative density is 1.08 (60°C), add ethanol to make the alcohol content in the solution 70% (v / v), stir well, heat to 60°C, let it stand for 24 hours, filter, recover ethanol from the filtrate and concentrate to a relative density of 1.20 (60°C) concentrated solution (concentrated solution A); then take...
Embodiment 2
[0080] Ligusticum chuanxiong 555g, extract volatile oil by steam distillation for later use, or wrap the extracted volatile oil with β-cyclodextrin of 4 times the weight of volatile oil to synthesize clathrate, dry, and pulverize (to obtain volatile oil inclusion complex powder); Combine the residue with 835g of Pueraria lobata root, add water and decoct twice, 1 hour each time, the amount of water used is 10 and 8 times the total weight of Rhizoma Chuanxiong and Pueraria lobata root, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.10 (60°C) , add ethanol so that the alcohol content in the solution is 75%, stir evenly, heat to 60°C, let stand for 48h, filter, recover ethanol from the filtrate and concentrate it into a concentrated solution (concentrated solution A) with a relative density of 1.25 (60°C); Then take 555g of Salvia miltiorrhiza and 555g of Erigeron breviscapine and add 80% ethanol to heat and reflux to extract twice, each tim...
Embodiment 3
[0081] Embodiment 3: tablet
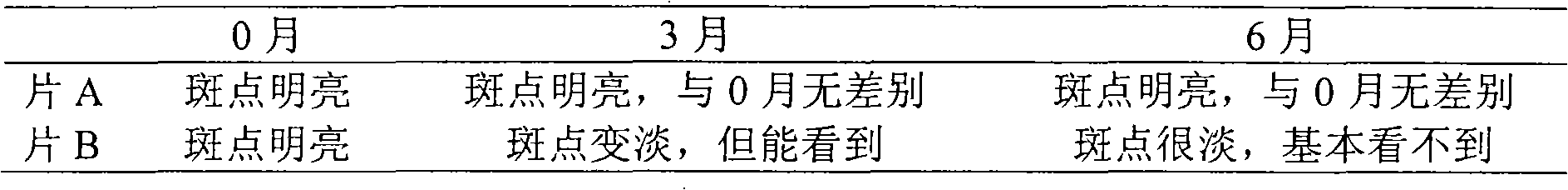
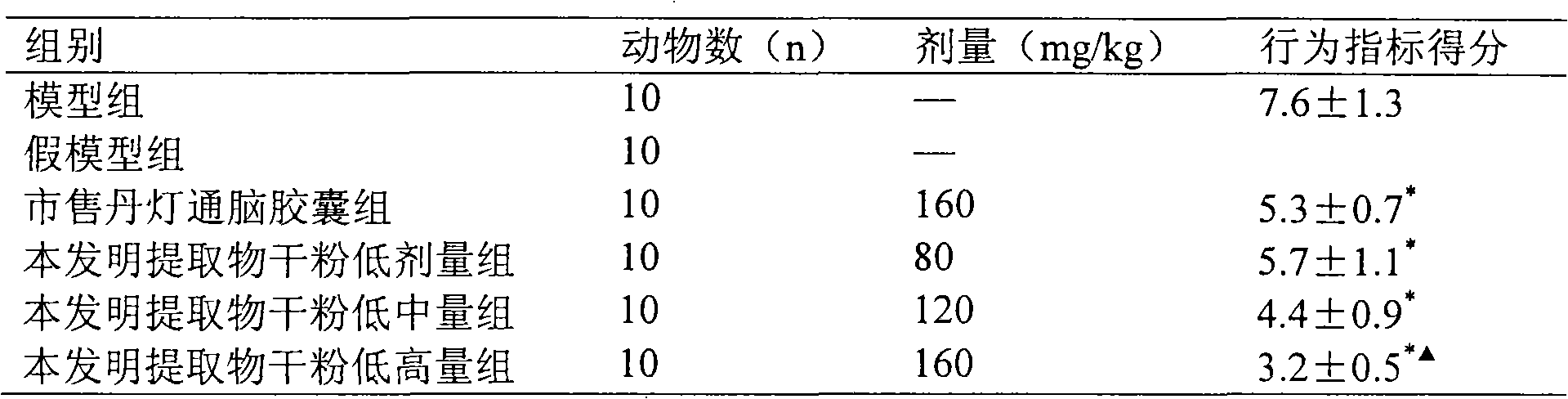
[0082] Add 240 grams of starch to dry powder of the extract obtained in Example 1 or 2, mix well, pass through a No. 5 sieve, granulate with 95% ethanol, dry below 60°C, granulate, add 0.5% magnesium stearate, mix well , pressed into 1000 tablets, each 0.5g, coated with film, is the tablet of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com