Real-time PCR system
A technology of polymerase and chain reaction, which is applied in the direction of laboratory equipment, biochemical instruments, biochemical equipment and methods, etc., can solve the problems of uncontrollable amplification level, infeasible comprehensive analysis, and inability to analyze together at the same time, so as to achieve high precision The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] Hereinafter, preferred embodiments of the real-time PCR system according to the present invention are described with reference to the accompanying drawings. It should be noted that the drawings show preferred embodiments of the invention by way of example, and the invention should not be construed narrowly by the preferred embodiments.
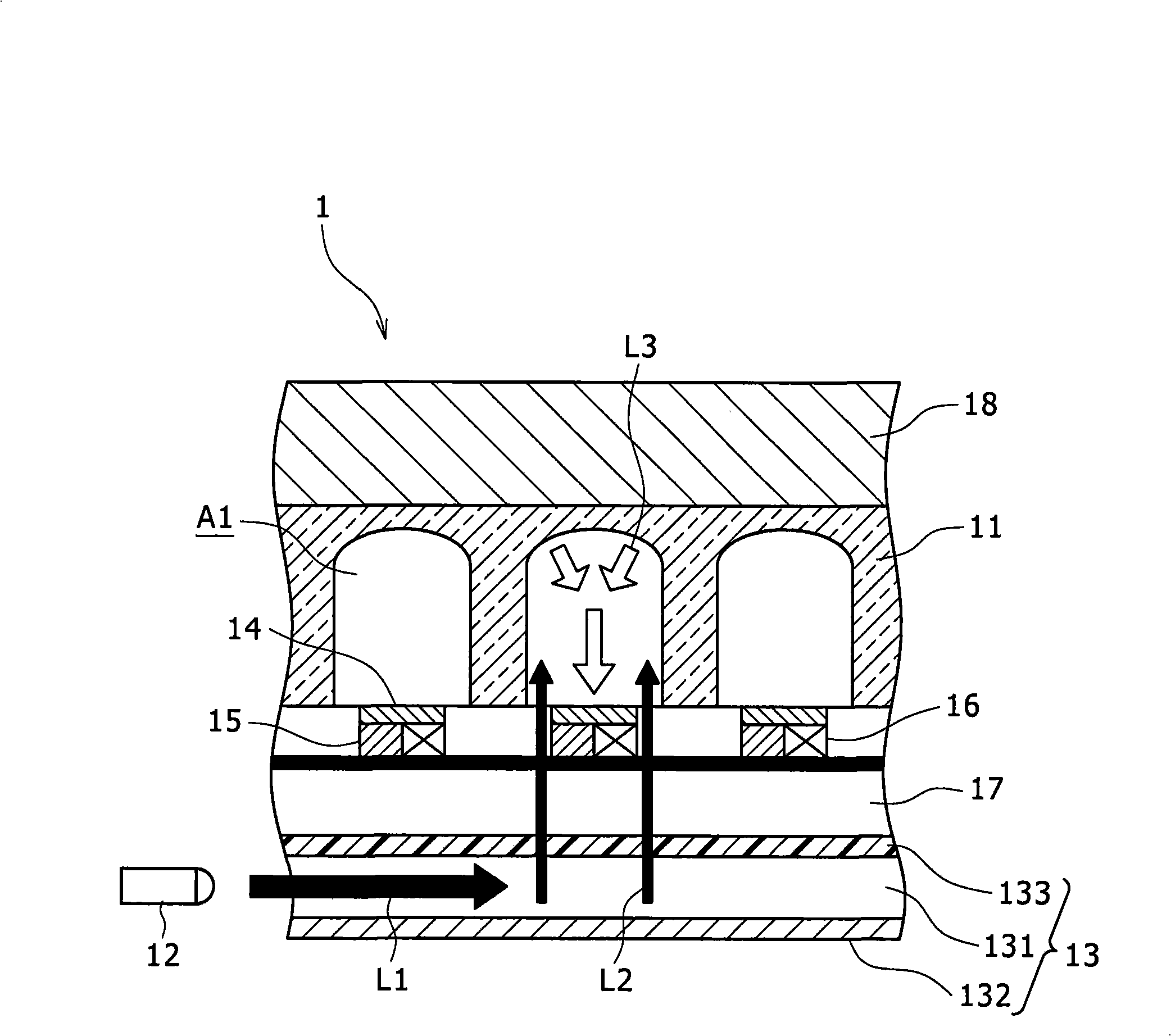
[0034] First, refer to figure 1 , the first embodiment of the real-time PCR system according to the present invention will be described hereinafter.
[0035] figure 1 Reference number 1 in indicates the first embodiment of the real-time PCR according to the present invention. As desired, the size and layer structure of the real-time PCR system 1 can be selectively determined according to the purpose of application. As long as the real-time PCR system 1 can achieve the purpose of the present invention, the configuration of the real-time PCR system 1 can also be designed or improved as required.
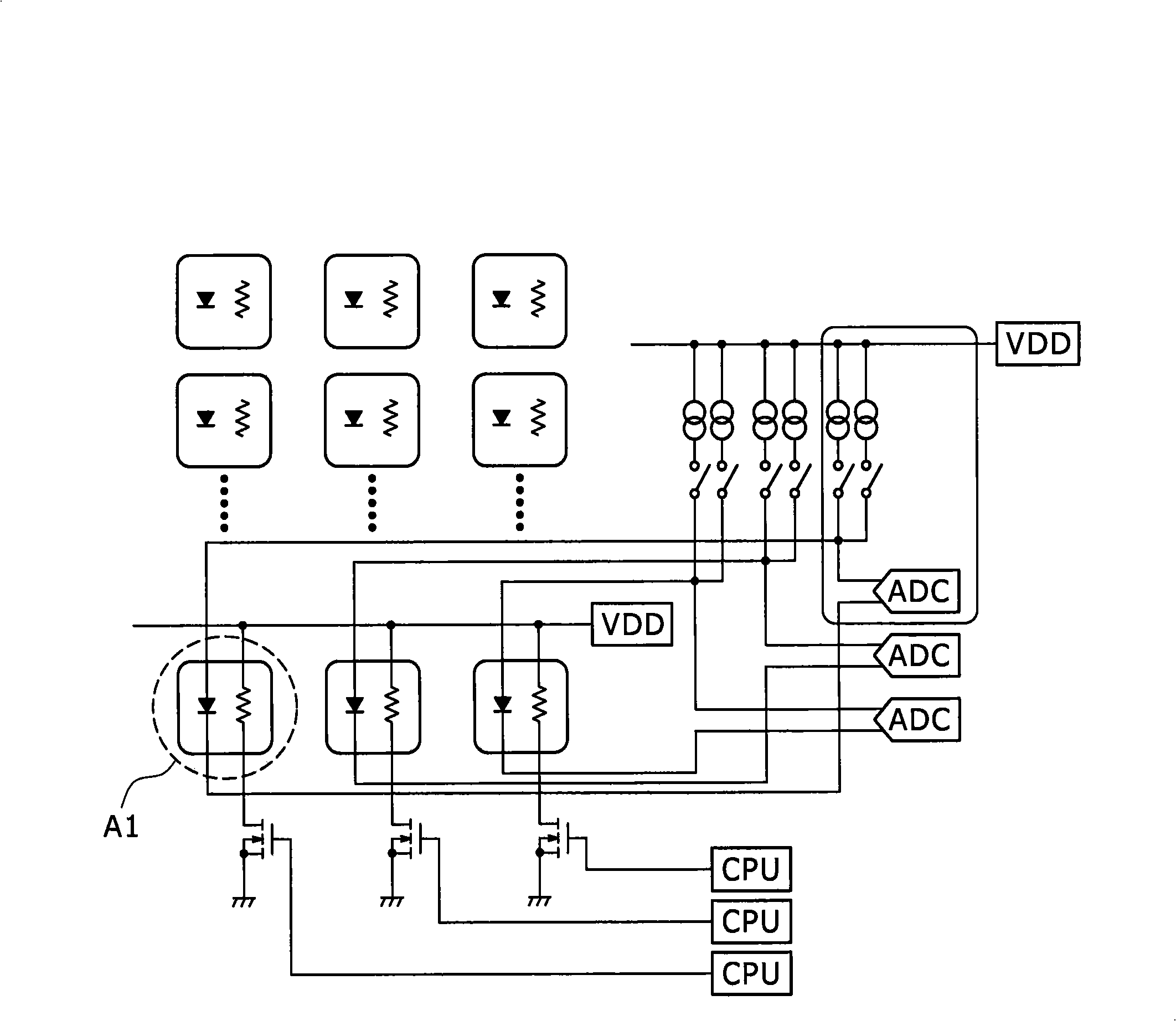
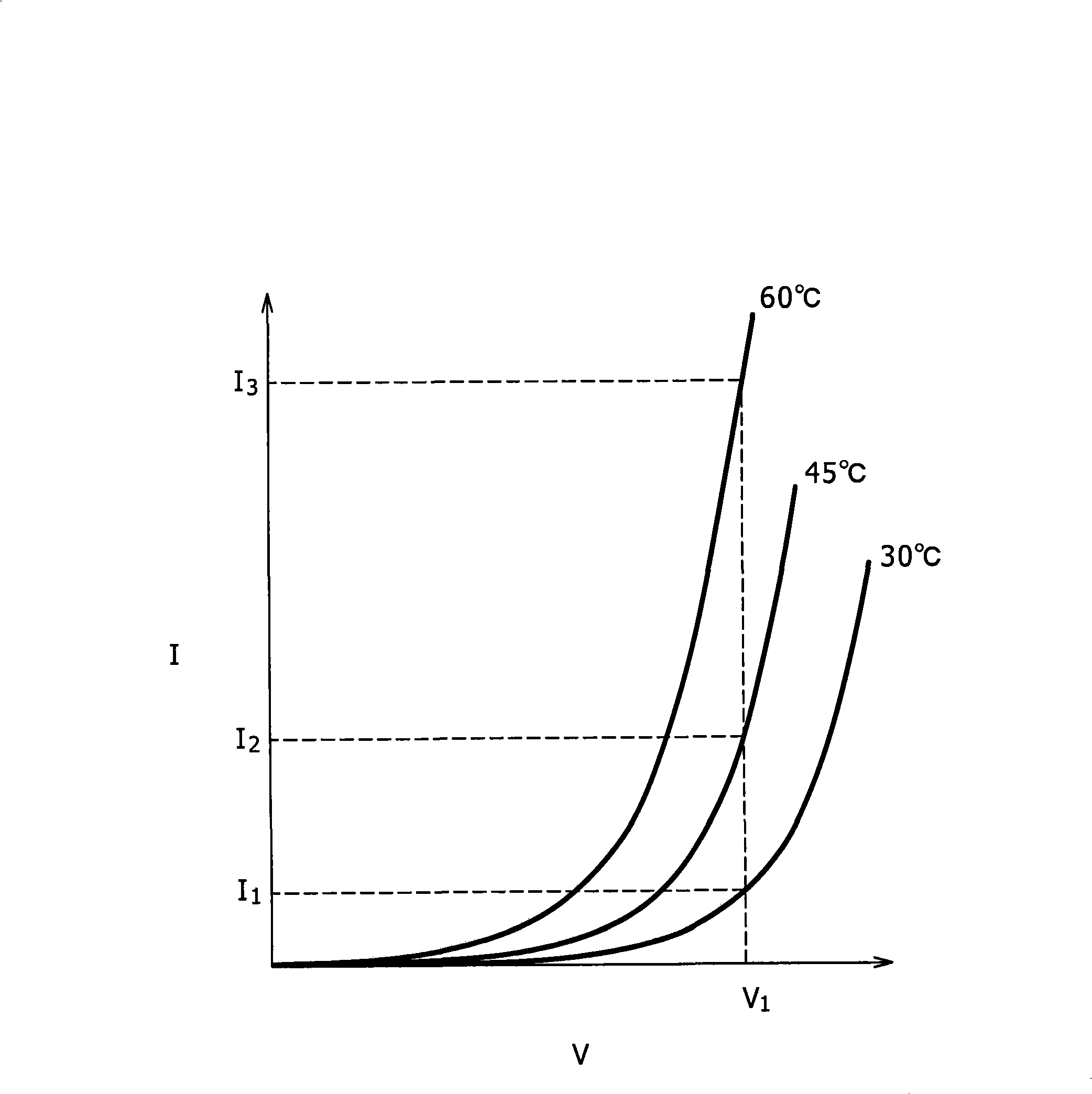
[0036] The real-time PCR system 1 is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com