Process for recovering rare earth element in waste florescent lamps
A technology of rare earth elements and fluorescent lamps, applied in the direction of improving process efficiency, etc., can solve the problem of not realizing the separation of rare earth elements and other valuable elements, and achieve the effect of reasonable process flow, economical and practical process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
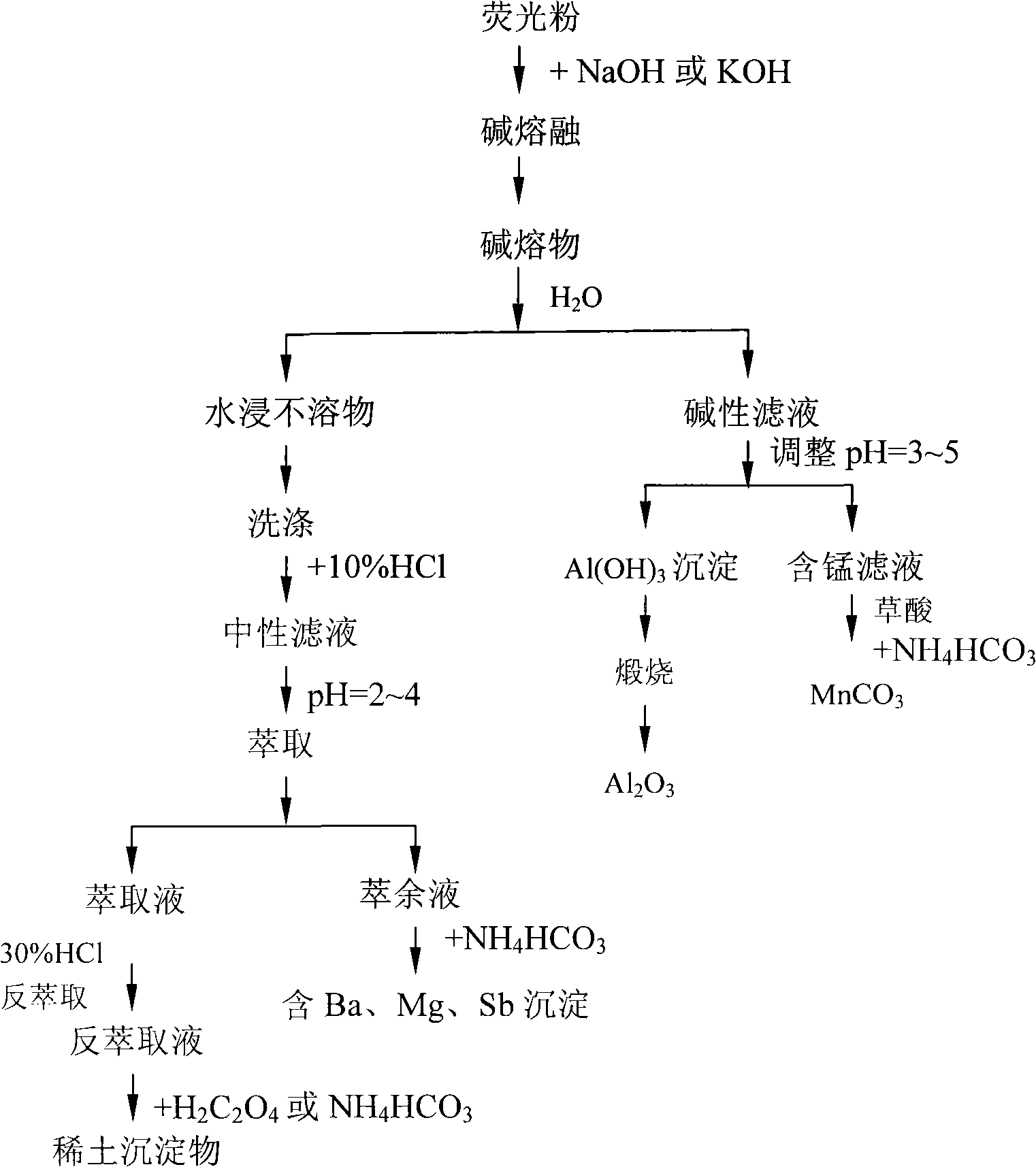
[0015] Take 100g waste mixed fluorescent powder, 500g NaOH, mix evenly, and alkali melt at 320°C for 2 hours to obtain alkali melt. The alkali melt was stirred and leached with 1000g of water, filtered and washed to obtain 62.5g of water-insoluble matter and alkaline filtrate. Water insolubles are dissolved with 10% hydrochloric acid, filtered to obtain a neutral filtrate, adjust the pH of the neutral filtrate=4, and use P 204 Extraction and separation of rare earth containing Y, Ce, Eu, Tb, Mg, Ba, Sb neutral filtrate to obtain extract and raffinate, extract the extract with 30% HCl to obtain strip extract, and use NH 4 HCO 3 Precipitate, and obtain 60.2g mixed Y, Ce, Tb, Eu rare earth precipitates. Raffinate with NH 4 HCO 3 Precipitate, and obtain 12.3 g of precipitates containing Ba, Mg, and Sb. Adjust the pH of the alkaline filtrate to 3.8, and filter to obtain a manganese-containing filtrate and aluminum hydroxide precipitate. Wash, filter, and calcinate the aluminu...
Embodiment 2
[0017] Take 100g waste mixed fluorescent powder, 300g NaOH, mix evenly, and alkali melt at 400°C for 6 hours to obtain alkali melt. The alkali melt was stirred and leached with 1000g of water, filtered and washed to obtain 68.5g of water-insoluble matter and alkaline filtrate. Dissolve the insolubles in water with 10% hydrochloric acid, filter to obtain a neutral filtrate, adjust the pH of the neutral filtrate to 2.5, and use P 507 Extraction and separation of rare earth containing Y, Ce, Eu, Tb, Mg, Ba, Sb neutral filtrate to obtain extract and raffinate, back-extract the extract with 20% HCl to obtain back-extraction, back-extract with H 2 C 2 o 4 Precipitate, and obtain 88.2g mixed Y, Ce, Tb, Eu rare earth precipitates. Raffinate with NH 4 HCO 3 Precipitate, and obtain 10.5 g of precipitates containing Ba, Mg, and Sb. Adjust the pH of the alkaline filtrate to 5, and filter to obtain a manganese-containing filtrate and aluminum hydroxide precipitate. Wash, filter, and...
Embodiment 3
[0019] Take 100g waste mixed fluorescent powder, 300g KOH, mix evenly, and alkali melt at 500°C for 10 hours to obtain alkali melt. The alkali melt was stirred and leached with 1000g of water, filtered and washed to obtain 68.5g of water-insoluble matter and alkaline filtrate. Water insolubles are dissolved with 10% hydrochloric acid, filtered to obtain a neutral filtrate, adjust the pH of the neutral filtrate to 3.2, and use P 507 Extraction and separation of rare earth containing Y, Ce, Eu, Tb, Mg, Ba, Sb neutral filtrate to obtain extract and raffinate, back extract the extract with 10% HCl to obtain back extract, back extract with NH 4 HCO 3Precipitation, 62g mixed Y, Ce, Tb, Eu rare earth precipitates were obtained. Raffinate with NH 4 HCO 3 Precipitate, and obtain 13.7g of precipitates containing Ba, Mg, and Sb. Adjust the pH of the alkaline filtrate to 3 and filter to obtain a manganese-containing filtrate and aluminum hydroxide precipitate. Wash, filter, and calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com