Antiviral protein and uses thereof
A protein and anti-virus technology, applied in the protein field, can solve problems such as inability to encode viral proteins, G→A high-frequency mutation, truncated non-functional products, etc., to reduce immunogenicity, improve membrane penetration efficiency, facilitate purification or Detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
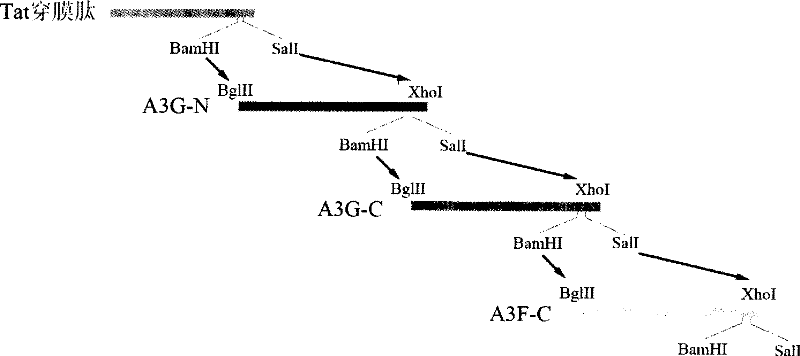
[0038] Example 1 Preparation of antiviral protein
[0039] The amino acid sequence of the antiviral protein of the present embodiment is:
[0040] MetGlySerSer HisHisHisHisHisHis SerSerGlyLeuValProArgGlySerHisMetAlaSerMetThrGlyGlyGlnGlnMetGlyArgGlySer lyGlyGlyGlySerValAspLeuGlu HisHisHisHisHisHis
[0041]This antiviral protein contains a membrane-penetrating peptide domain (GlyArgLysLysArgArgGlnArgArgArgProProGln) and three cytosine deaminase domains derived from the APOBEC family (these three cytosine deaminase domains are sequentially derived from the cytosine at the N-terminal of the hAPOBEC3G molecule 脱氨酶结构域HisProGluMetArgPhePheHisTrpPheSerLysTrpArgLysLeuHisArgAspGlnGluTyrGluValThrTrpTyrIleSerTrpSerProCysThrLysCys、来自hAPOBEC3G分子C端的胞嘧啶脱氨酶结构域HisAlaGluLeuCysPheLeuAspValIleProPheTrpLysLeuAspLeuAspGlnAspTyrArgValThrCysPheThrSerTrpSerProCysPheSerCys、来自hAPOBEC3F分子C端的胞嘧啶脱氨酶结构域HisAlaGluArgCysPheLeuSerTrpPheCysAspAspIleLeuSerProAsnThrAsnTyrGluValThrTrpTyrThrSerTrpSerProCysProG...
Embodiment 2
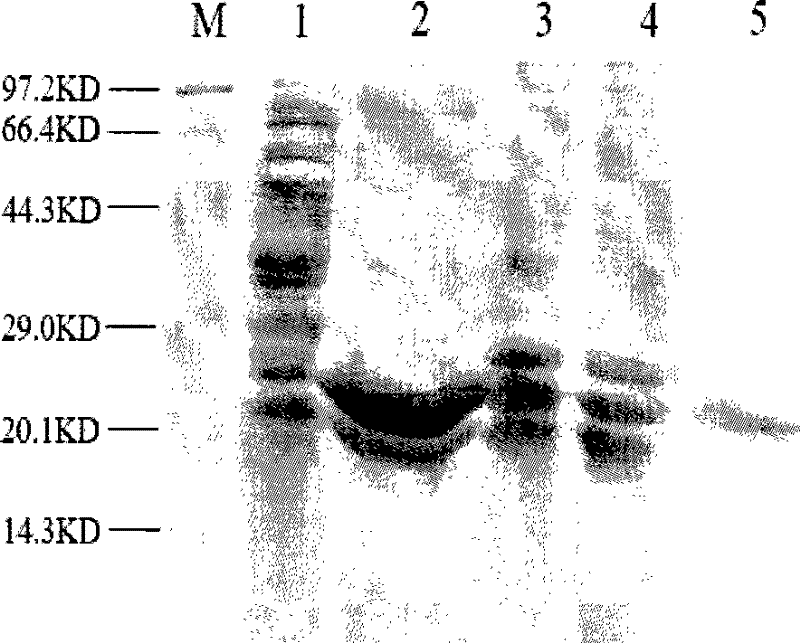
[0103] Example 2 Western blot detection of the antiviral protein prepared in Example 1
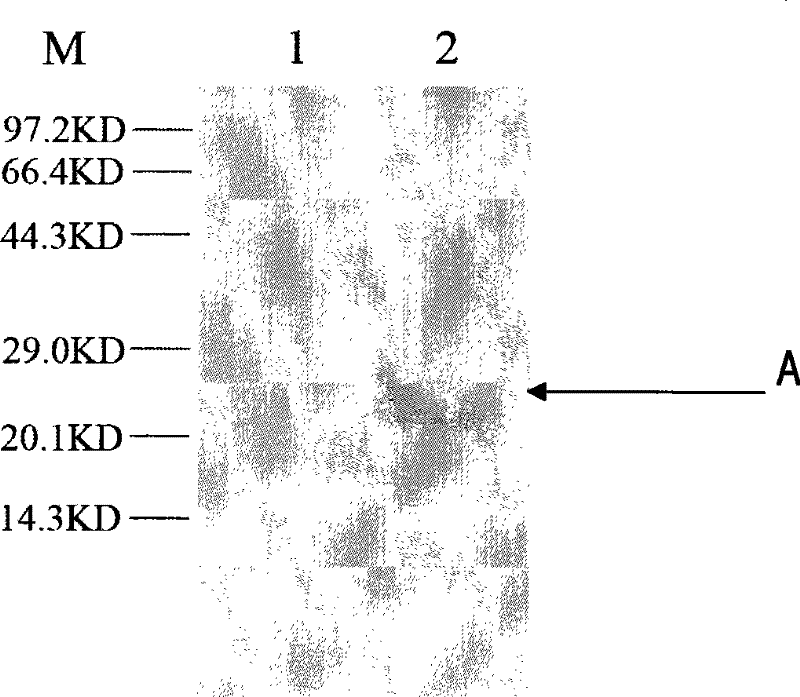
[0104] The purified antiviral protein was added to the HepG2.2.15 cells growing into a monolayer at a final concentration of 10 μg / ml (that is, the HepG2.2.15 cells growing into a monolayer finally contained 10 μg of purified antiviral protein), As the experimental group; add PBS to HepG2.2.15 cells in the control group. After 8 hours, the cells were lysed to extract the total protein, and Western blot was performed with an anti-His tag mouse monoclonal antibody, and detected by chemiluminescence. like image 3 ( image 3 Among them, M represents protein molecular weight standard; Swimming lane 1. Western blot result of HepG2.2.15 cells in the control group; Swimming lane 2. As shown in the Western blot result of HepG2.2.15 cells in the experimental group adding antiviral protein), the cells in the experimental group appeared at 22KD There was a distinct band A, consistent with the mole...
Embodiment 3
[0105] Example 3 Analysis of intracellular localization of the antiviral protein prepared in Example 1
[0106]Add the purified antiviral protein to the HepG2.2.15 cell slides growing into a monolayer at a final concentration of 5 μg / ml (that is, each ml of HepG2.2.15 cell slides growing into a monolayer contains 5 μg of purified antiviral protein) as the experimental group; add PBS to the HepG2.2.15 cell slides of the control group. After 8 hours, the cell slides were fixed with 4% paraformaldehyde at room temperature for 10 min; then 0.2% Triton-X100 (Triton-X100) was added, and fixed at room temperature for 5 min; then, anti-His tag The mouse monoclonal antibody was used as the primary antibody, and the Cy3-labeled goat anti-mouse antibody was used as the secondary antibody. Observed under a fluorescent microscope, such as Figure 4 as shown ( Figure 4 The middle red part shows the antiviral protein that enters the cytoplasm), and it was found that the antiviral protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com