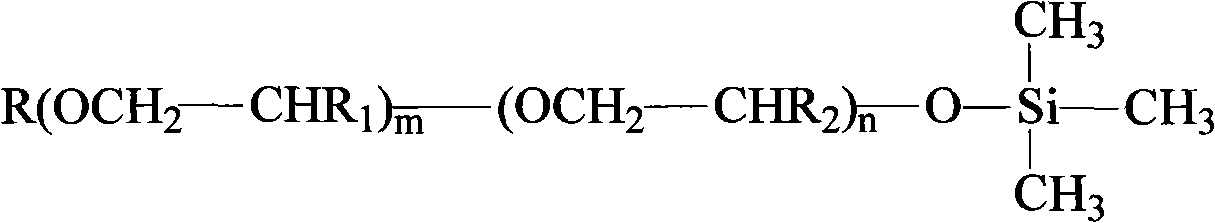End capping polyether prepared with end hydroxyl radical polyether and preparation thereof
A technology of hydroxyl-terminated polyether and capped polyether, which is applied in the field of capped polyether and its preparation, and can solve problems such as low capping rate and unstable capping rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add allyl alcohol polyether (its structure is CH 2 =CH-CH 2 -(OCH 2 CH 2 ) 10 -(OCH 2 CHCH 3 ) 4 OH) 350g, add 45g of sodium hydroxide, and add co-solvent methanol 100ml, react at -0.08~-0.10Mpa, 85°C for 2.5 hours; cool down to 60°C, dropwise add trimethylchlorosilane 83g, about 1 hour After the dropwise addition is completed, the reaction is continued for 1.5 hours; the pH value of the reaction product is adjusted to 5-6 with acid, and diatomaceous earth and polyether refining preparation are added for treatment for 2 hours to obtain a blocked polyether.
[0021] The end-capped polyether prepared in this example, the end-capping rate is shown in Table 1 through infrared test analysis.
Embodiment 2
[0023] Add allyl alcohol polyether (its structure is CH 2 =CH-CH 2 -(OCH 2 CH 2 ) 16 -(OCH 2 CHCH 3 ) 7 OH) 500g, add 25g of sodium hydroxide, and 50ml of co-solvent methanol, react at -0.08~-0.10Mpa, 80°C for 3.0 hours; cool down to 60°C, dropwise add trimethylchlorosilane 60g, about 40 minutes After the dropwise addition is completed, the reaction is continued for 1.0 hour; the pH value of the reaction product is adjusted to 5-6 with acid, and diatomaceous earth and polyether refining preparation are added for treatment for 2 hours to obtain a blocked polyether.
[0024] The end-capped polyether prepared in this example, the end-capping rate is shown in Table 1 through infrared test analysis.
Embodiment 3
[0026] Add allyl alcohol polyether (its structure is CH 2 =CH-CH 2 -(OCH 2 CH 2 ) 10 -(OCH 2 CHCH 3 ) 4 OH) 350g, add 40g sodium methoxide, and add 100ml co-solvent methanol, react at -0.08~-0.10Mpa, 120°C for 2.5 hours; cool down to 60°C, drop trimethylchlorosilane 80g, about 1 hour After the addition is completed, the reaction is continued for 1.5 hours after the dropwise addition; the pH value of the reaction product is adjusted to 5-6 with an acid, and diatomaceous earth and polyether refining preparation are added for treatment for 2 hours to obtain a blocked polyether.
[0027] The end-capped polyether prepared in this example, the end-capping rate is shown in Table 1 through infrared test analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com