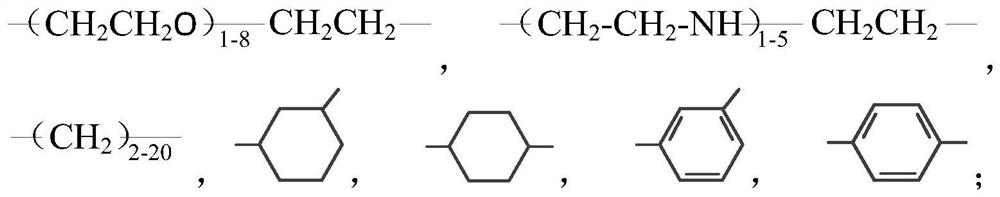
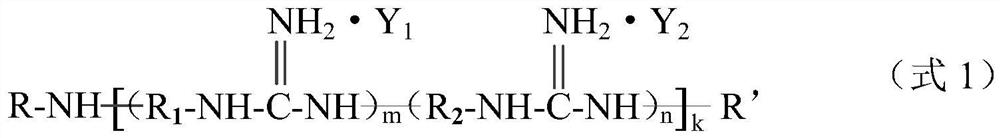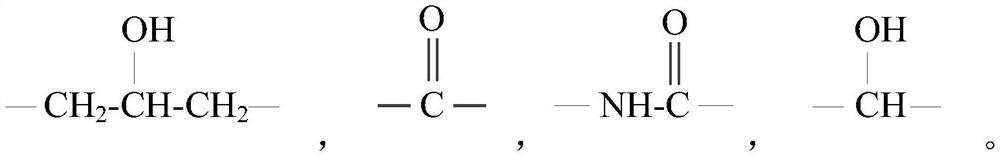Guanidine salt polymer as well as preparation method and application thereof
A guanidine salt polymer and a guanidine salt technology are applied in the field of guanidine salt polymer and its preparation, which can solve the problems of adverse effects on the use performance of anti-harmful microbial materials, low bonding and end-capping efficiency, low reactivity, etc., and achieve improved application The effect of value, good product safety, flexible overall performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Get 38.2g guanidine hydrochloride (0.4mol) and 62.8g guanidine phosphate (0.4mol), join respectively In two three-necked flasks. Then add 54.5g m-xylylenediamine (0.4mol) in the flask containing guanidine hydrochloride, add 46.5g 1,6-hexamethylenediamine (0.4mol) in the flask containing guanidine phosphate, under nitrogen protection, stir and The temperature was raised to 90°C, and the reaction was carried out for 2.5 hours. Then the materials in the two flasks were combined in one flask, and the temperature was gradually raised to 170°C to continue the reaction for 2 hours. Finally, the temperature was lowered to 158°C, and 38.8 g of dodecyl glycidyl ether (0.16 mol) was added dropwise within 1.5 hours, then reacted for another 0.5 hour, and poured out while hot to obtain guanidinium salt polymer-1.
Embodiment 2
[0071] Get 62.8g guanidine phosphate (0.4mol) and 68.7g guanidine stearate (0.2mol), join respectively in two three-necked flasks. Then add 54.5g m-xylylenediamine (0.4 mol) in the flask containing guanidine phosphate, add 23.2g 1,6-hexamethylenediamine (0.2mol) in the flask containing guanidine stearate, under nitrogen protection, Stir and heat up to 105°C, and react for 2 hours. Then the materials in the two flasks were combined in one flask, and the temperature was gradually raised to 175° C. to continue the reaction for 3 hours. Finally, the temperature was lowered to 155°C, and a total of 14.8 g of phthalic anhydride (0.1 mol) was added in 4 times within 1 hour, then reacted for another 0.5 hour, and poured out while hot to obtain guanidinium salt polymer-2.
Embodiment 3
[0073] Get 48.4g guanidine carbonate (0.4mol) and 137.4g guanidine stearate (0.4mol), join in two three-necked flasks respectively. Then add 40.8g pentamethylenediamine (0.4mol) in the flask containing guanidine carbonate, also add 40.8g pentamethylenediamine (0.4mol) in the flask containing guanidine stearate, under nitrogen protection, stir and be warming up to 95 °C, react for 1.5 hours. Then the materials in the two flasks were combined in one flask, and the temperature was gradually raised to 170° C. to continue the reaction for 3 hours. Finally, the temperature was lowered to 150°C, and 26 g of butyl glycidyl ether (0.2 mol) was added dropwise within 1.5 hours, then reacted for another 0.5 hour, and poured out while hot to obtain guanidine salt polymer-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com