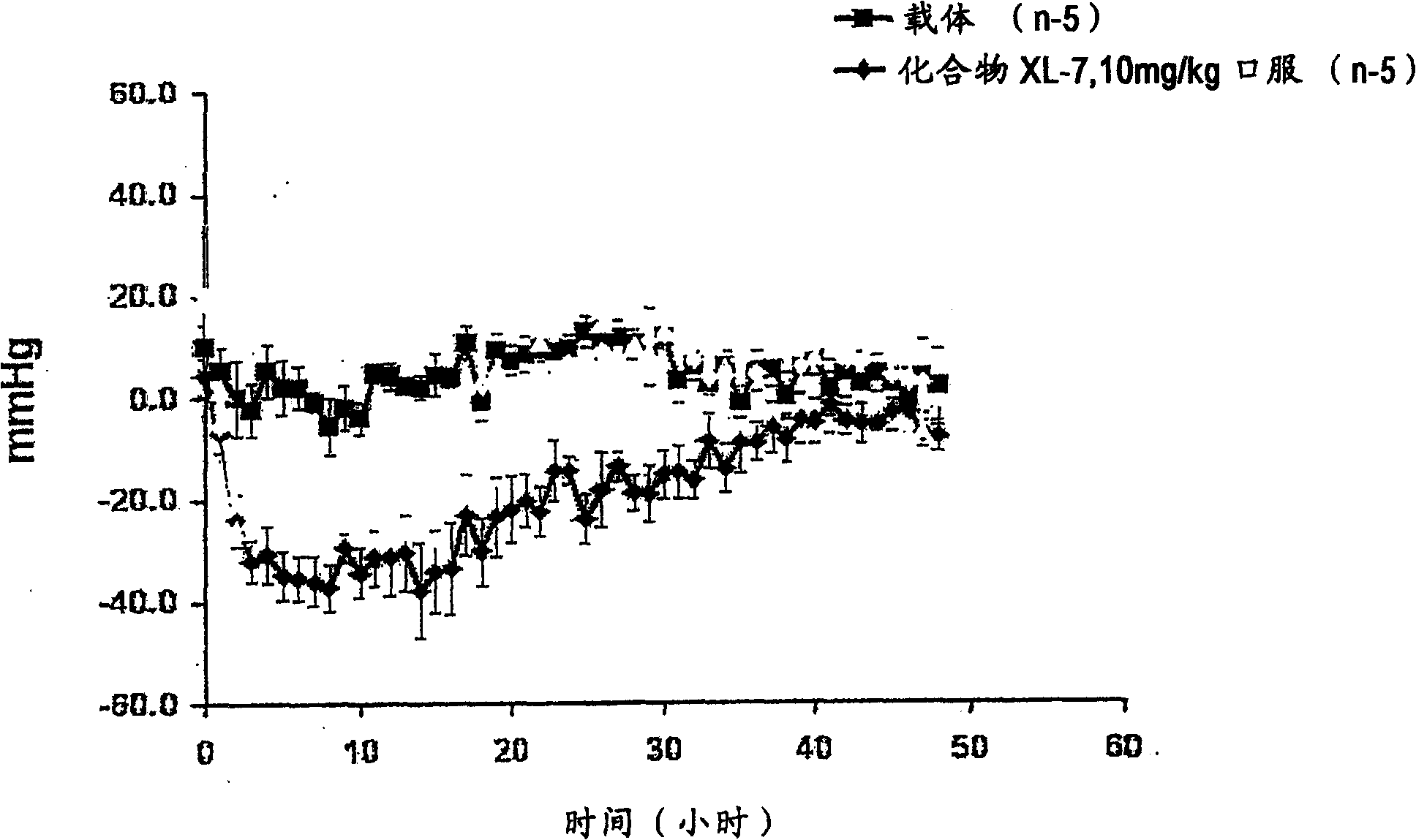
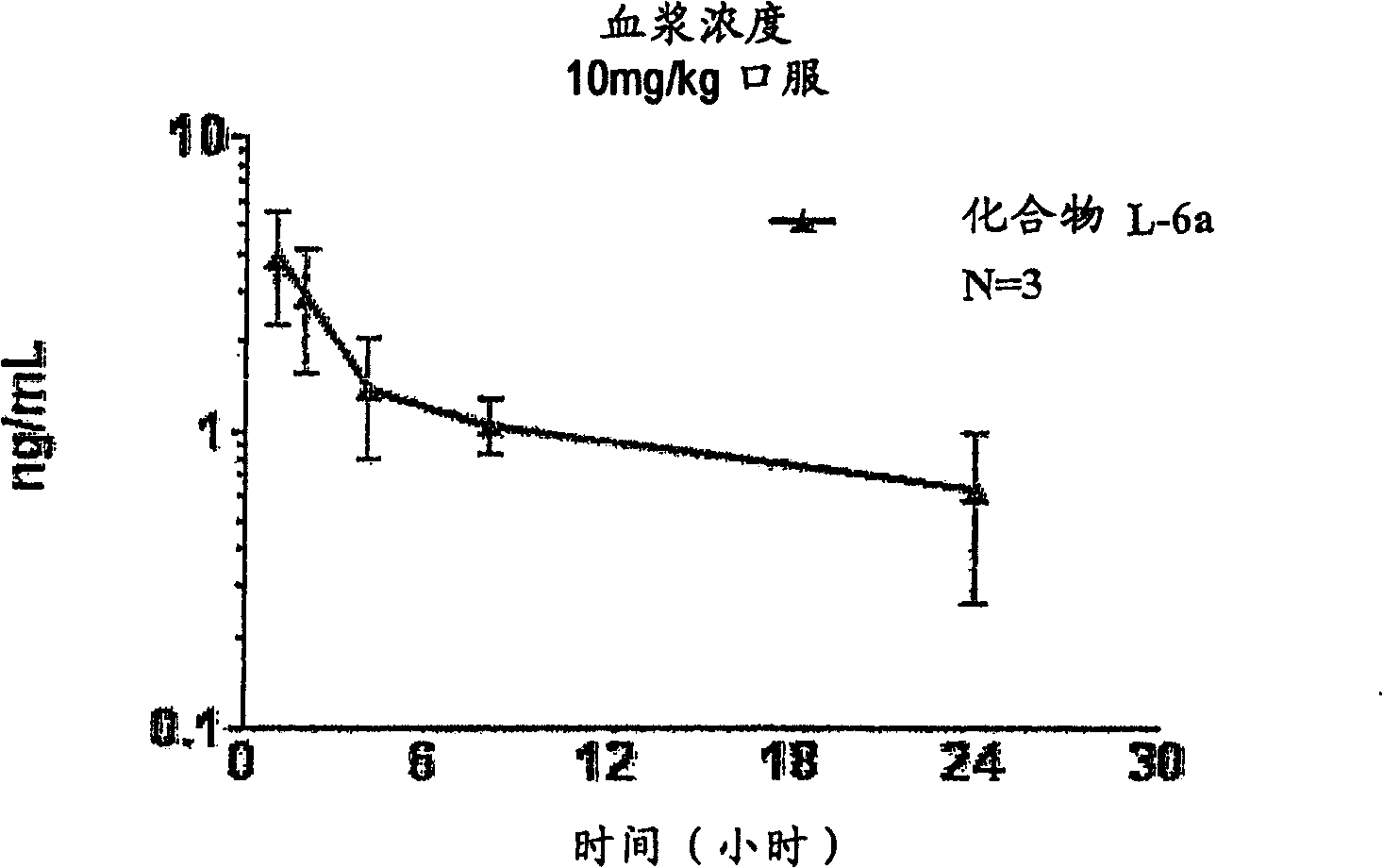
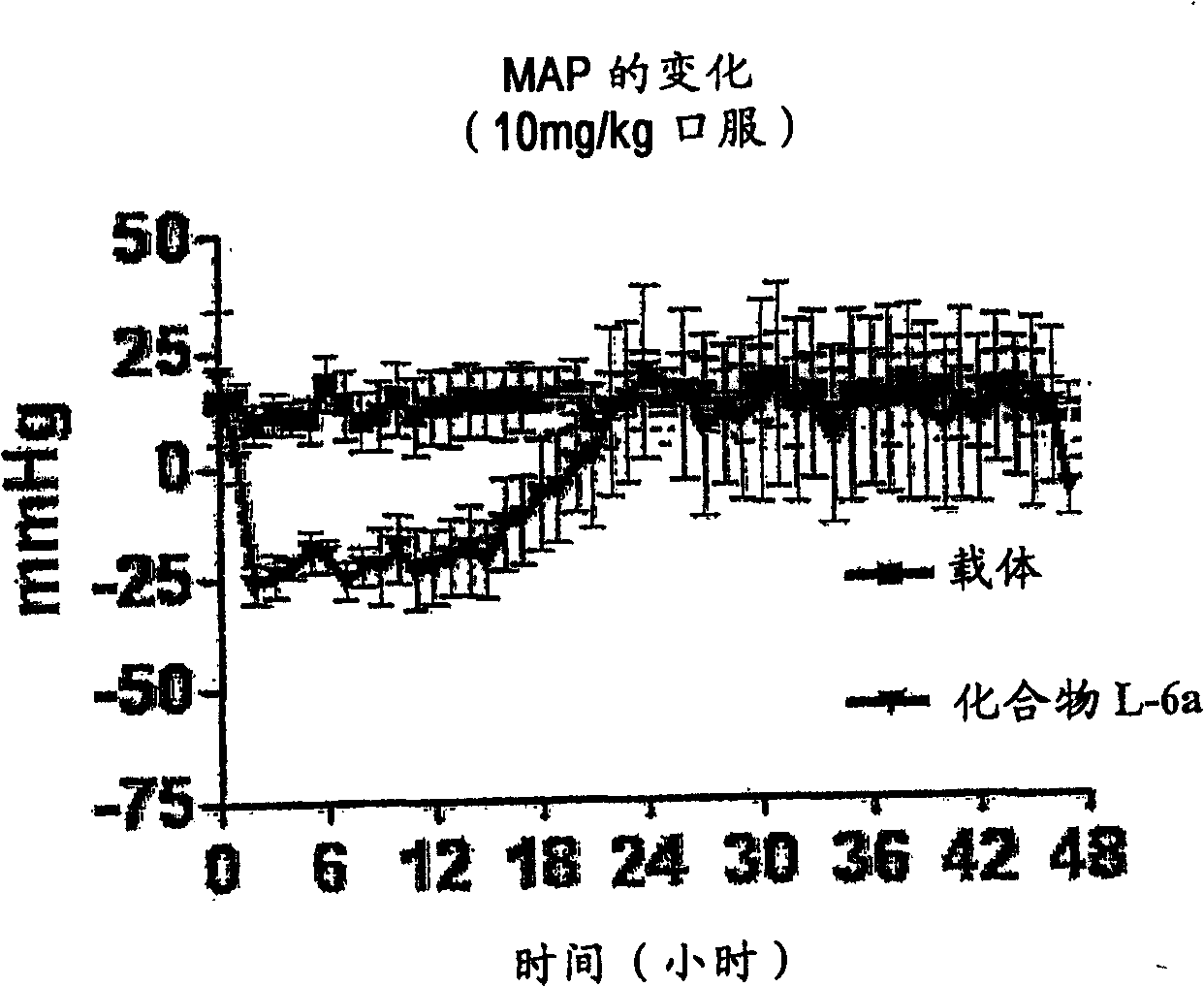
Aspartic protease inhibitors
A representative and compound technology, applied in the field of aspartic protease inhibitors, can solve the problem of not obtaining metabolic renin inhibitors and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0369] Preparative HPLC refers to preparative reversed-phase HPLC on a C-18 column, eluting with a gradient of water / acetonitrile containing 0.01% TFA, and running on a Gilson 215 system.
[0370] Analytical method
[0371] LC-MS (3 minutes)
[0372] Column: Chromolith SpeedRod, RP-18e, 50×4.6mm; mobile phase: A: 0.01% TFA / water, B: 0.01% TFA / CH 3 CN; Flow rate: 1mL / min; Gradient:
[0373] Time (minutes)
[0374] Intermediate preparation example
preparation example 1
[0376] (S)-4-(3-chlorophenyl)-4-hydroxy-4-((R)-piperidin-3-yl)butylcarbamate (31)
[0377]
[0378] Step 1. 3-(Methoxy(methyl)carbamoyl)piperidine-1-carboxylic acid (R)-tert-butyl ester
[0379] To a stirred solution of (R)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid (233 g, 1.2 mol) in THF (1.2 L) was added carbonyl diimidazole (230 g, 1.42 mol). The mixture was stirred in an ice water bath for 1 hour. A suspension of triethylamine (207 mL, 1.41 mol) and N,O-dimethylhydroxylamine hydrochloride (138 g, 1.42 mol) in THF (900 mL) was added. The reaction mixture was allowed to warm to room temperature and stirred overnight. After tlc showed that the reaction was complete, the solvent was evaporated. Dissolve the residue in CH 2 Cl 2 (1.2L), use 0.5N HCl aqueous solution, saturated Na 2 CO 3 Washed with aqueous solution and brine, dried over anhydrous sodium sulfate, and evaporated to give 3-(methoxy(methyl)-carbamoyl)piperidine-1-carboxylic acid (R)-tert-butyl ester (250g, ...
preparation example 2
[0393] (R)-2-((3-Chloro-2-fluorophenyl)(piperidin-3-yl)methoxy)acetamide
[0394]
[0395] Step 1. 3-(3-Chloro-2-fluorobenzoyl)piperidine-1-carboxylic acid (R)-tert-butyl ester
[0396] To a stirred solution of 1-chloro-2-fluoro-benzene (13.0g, 0.1mol) in THF (250mL) was added dropwise 2.5M BuLi in hexane (40mL, 0.1 mol). After stirring for an additional 30 minutes at -75°C, 3-(methoxy(methyl)-carbamoyl)piperidine-1-carboxylic acid (R)-tert-butyl ester (21.76g , 0.08 mol) in THF (100 mL). The mixture was warmed from -70°C to 0°C. The mixture is saturated with NH 4 The aqueous Cl solution was quenched, extracted with EtOAc (3x), and the combined organic layer was washed with Na 2 SO 4 dry. The solvent was removed, flash column chromatography was performed, and washed with 5% EtOAc in petroleum ether to obtain 3-(3-chloro-2-fluorobenzoyl)piperidine-1-carboxylic acid (R)-tert-butyl ester (19.2g , 70%). 1 HNMR(400MHz, CDCl 3 ): 1.45 (s, 9H), 1.63 (m, 2H), 1.76 (m, 1H), 2.06 (m, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com