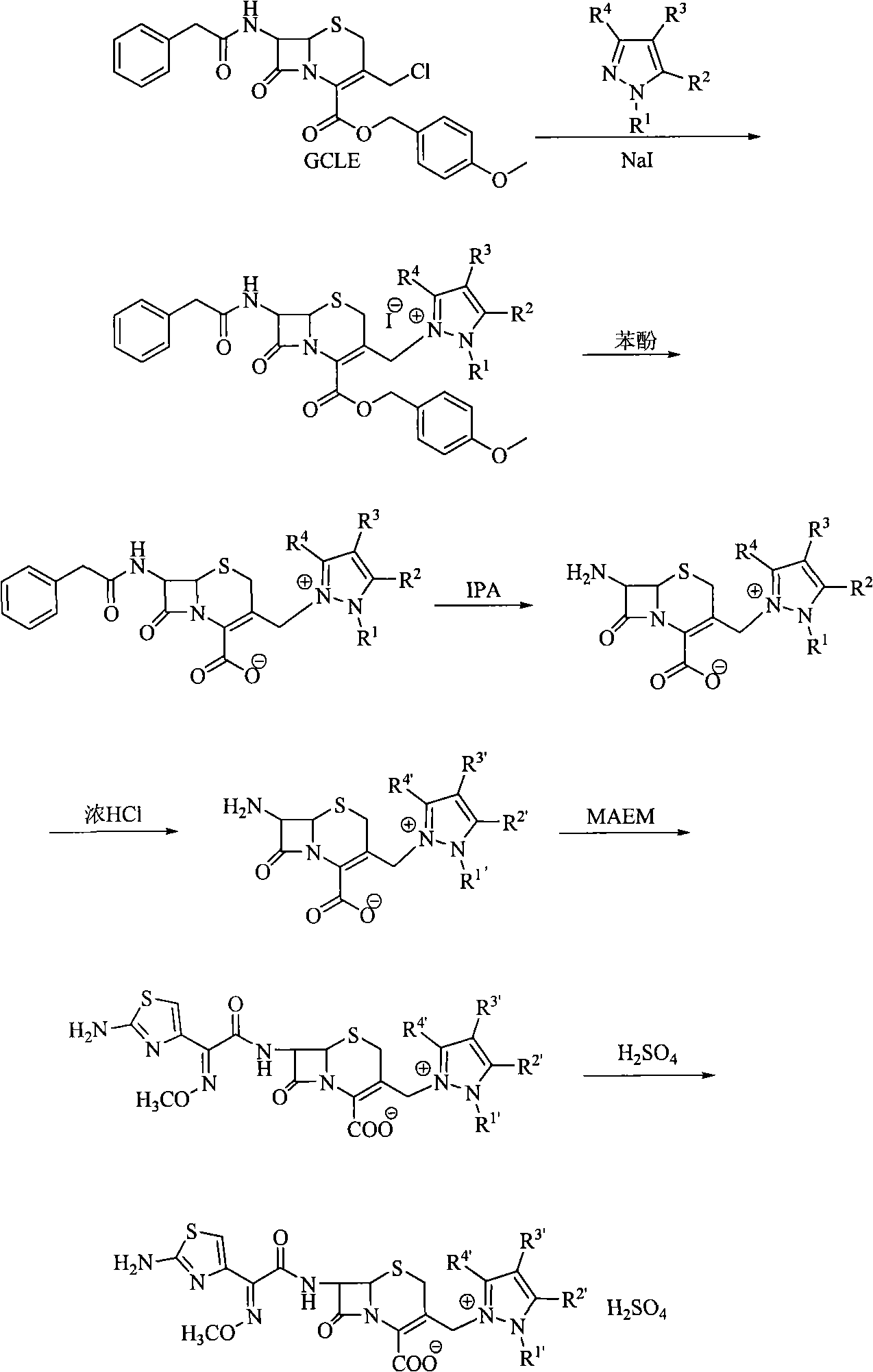
Novel technique for synthesizing cefoselis sulfate
A cefotaxime sulfate, a new process technology, applied in the direction of organic chemistry, etc., can solve the problems of difficulty in purchasing cephalosporin nuclei raw materials, limited application prospects, harsh reaction conditions, etc., and achieve low cost, simple synthesis process route, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, the synthesis of cefotaxime sulfate:
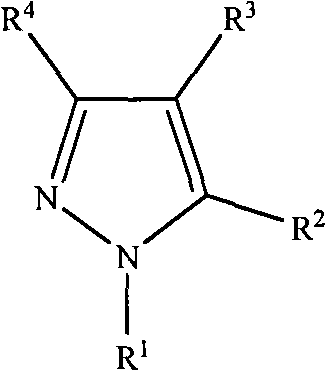
[0020] (1) Iodide 7β-phenylacetamido-3-[3-carboxamido-2-(2-formyloxyethyl)-pyrazolium]methyl-3-cephem-4-carboxylic acid pair Synthesis of Methoxybenzyl:
[0021] Dissolve 80 grams of GCLE and 30 grams of sodium iodide in 90 mL of DMF, stir and react at room temperature for 30 minutes in the dark under nitrogen protection, add 94 grams of 5-formamido-1-(2-formyloxyethyl)-pyrazole, and perform a high-efficiency liquid phase The reaction process was monitored by chromatography; after the reaction was complete, a small amount of acetone was added, and then added to stirred water to precipitate a precipitate, which was filtered and washed with water. vacuum drying;
[0022] (2) Synthesis of 7β-phenylacetamido-3-[3-carboxamido-2-(2-formyloxyethyl)-pyrazolium]methyl-ceph-3-ene-4-carboxylic acid :
[0023] Mix 80.0 g of the above-mentioned compound and 93.2 g of phenol and stir at 50° C., react, follow the reaction by hig...
Embodiment 2
[0030] Embodiment 2, cefotaxime sulfate analogue obtains synthesis:
[0031] (1) Synthesis of 7β-phenylacetamido-3-[1-(2-ethyl)-pyrazolium]methyl-ceph-3-ene-4-carboxylic acid:
[0032] Dissolve 3.3 grams of GCLE and 30.8 grams of sodium iodide in DMF90mL, stir and react at room temperature in the dark for 30 minutes, add 50 grams of ethylpyrazole, and monitor the reaction process by high performance liquid chromatography; after the reaction is complete, add a small amount of acetone, and then add to In the stirred water, a precipitate precipitated out, filtered and washed with water. vacuum drying;
[0033] (2) Synthesis of 7β-phenylacetamido-3-[1-(2-ethyl)-pyrazolium]methyl-ceph-3-ene-4-carboxylic acid:
[0034] Mix 43.5 grams of the above-mentioned compound and 60 grams of phenol and stir at 50°C, react, and follow the reaction with high performance liquid chromatography. After the reaction is complete, add acetone, and then add isopropyl ether to precipitate, filter, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com