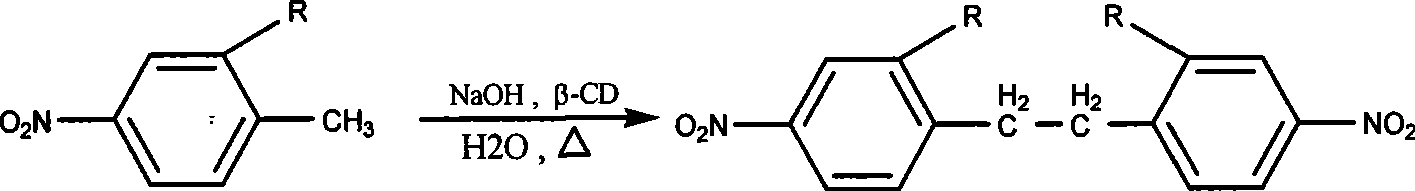
Preparation method of 4,4'-dinitrobibenzils compound
A dinitrobibenzyl compound technology, applied in 4 fields, can solve problems such as difficult separation, difficult storage, complicated operation, etc., and achieve the effects of reducing environmental pollution, reducing experimental costs, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 4, the preparation of 4'-dinitrobibenzyl
[0021] (1) Add 1.42g (1.25mmol) β-cyclodextrin, 2.2g sodium hydroxide and 10ml water successively in the reaction vessel equipped with electromagnetic stirrer, reflux condenser and thermometer, stir at room temperature until clarified, then Add 0.17g (1.25mmol) p-nitrotoluene, raise the temperature to about 55°C and react for 12h;
[0022] (2) Stop the reaction, place to precipitate, and filter with suction to obtain 0.15 g of yellow solid crude product;
[0023] (3) fully wash this solid 3 times with boiling hexanaphthene, to remove unreacted raw material;
[0024] (4) Extract the solid obtained in step (3) with boiling benzene for 3 times, discard the insoluble impurities, spin the gained benzene solution to obtain the product 4,4'-dinitrobibenzyl 0.1g, and the yield is 59 %. m.p.180~181℃, 1 HNMR (300MHz, CDCl 3 )δ: 8.142-8.114 (4H, d), 7.277-7.243 (4H, d), 3.058 (4H, s).
Embodiment 2
[0025] Example 2 Preparation of 2,2'-difluoro-4,4'-dinitrobibenzyl
[0026] (1) Add 1.42g (1.25mmol) β-cyclodextrin, 2.2g sodium hydroxide and 10ml water successively in the reaction vessel equipped with electromagnetic stirrer, reflux condenser and thermometer, stir at room temperature until clarified, then Add 0.2g (1.25mmol) 2-fluoro-4-nitrotoluene, raise the temperature to about 55°C and react for 4h;
[0027] (2) Stop the reaction, place to precipitate out, and filter with suction to obtain 0.21 g of yellow solid crude product;
[0028] (3) fully wash this solid 3 times with boiling hexanaphthene, to remove unreacted raw material;
[0029] (4) Extract the solid obtained in step (3) with boiling benzene for 3 times, discard the insoluble impurities, and spin the obtained benzene solution to obtain the product 2,2'-difluoro-4,4'-dinitrobi 0.14 g of benzyl, the yield is 70%. m.p.145~147℃, 1 HNMR (300MHz, CDCl 3 )δ: 7.942-7.878 (4H, t), 7.281-7.255 (2H, d), 3.059 (4H, s)...
Embodiment 3
[0030] Example 3 Preparation of 2,2'-dichloro-4,4'-dinitrobibenzyl
[0031] (1) Add 1.42g (1.25mmol) β-cyclodextrin, 2.2g sodium hydroxide and 10ml water successively in the reaction vessel equipped with electromagnetic stirrer, reflux condenser and thermometer, stir at room temperature until clarified, then Add 0.22g (1.25mmol) 2-chloro-4-nitrotoluene, raise the temperature to about 55°C and react for 6h;
[0032] (2) Stop the reaction, place to precipitate out, and filter with suction to obtain 0.2 g of yellow solid crude product;
[0033] (3) fully wash this solid 3 times with boiling hexanaphthene, to remove unreacted raw material;
[0034] (4) Extract the solid obtained in step (3) with boiling benzene for 3 times, discard the insoluble impurities, and spin the obtained benzene solution to obtain the product 2,2'-dichloro-4,4'-dinitrobi Benzyl 0.14g, the yield is 64%. m.p.161~163℃, 1 HNMR (300MHz, CDCl 3 )δ: 8.244 (2H, s), 8.028 ~ 8.001 (2H, d), 7.291 ~ 7.263 (2H, d)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com