Fusion proteins of tick anticoagulant peptide and staphylococcus aureus superantigen-like protein, preparation and application thereof
A technology of anticoagulant peptide and staphylococcus, which is applied in the direction of peptide/protein components, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve the problem of non-specific activation of the immune system and reduce troubles and pain, improve the level of prevention and treatment, and have good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
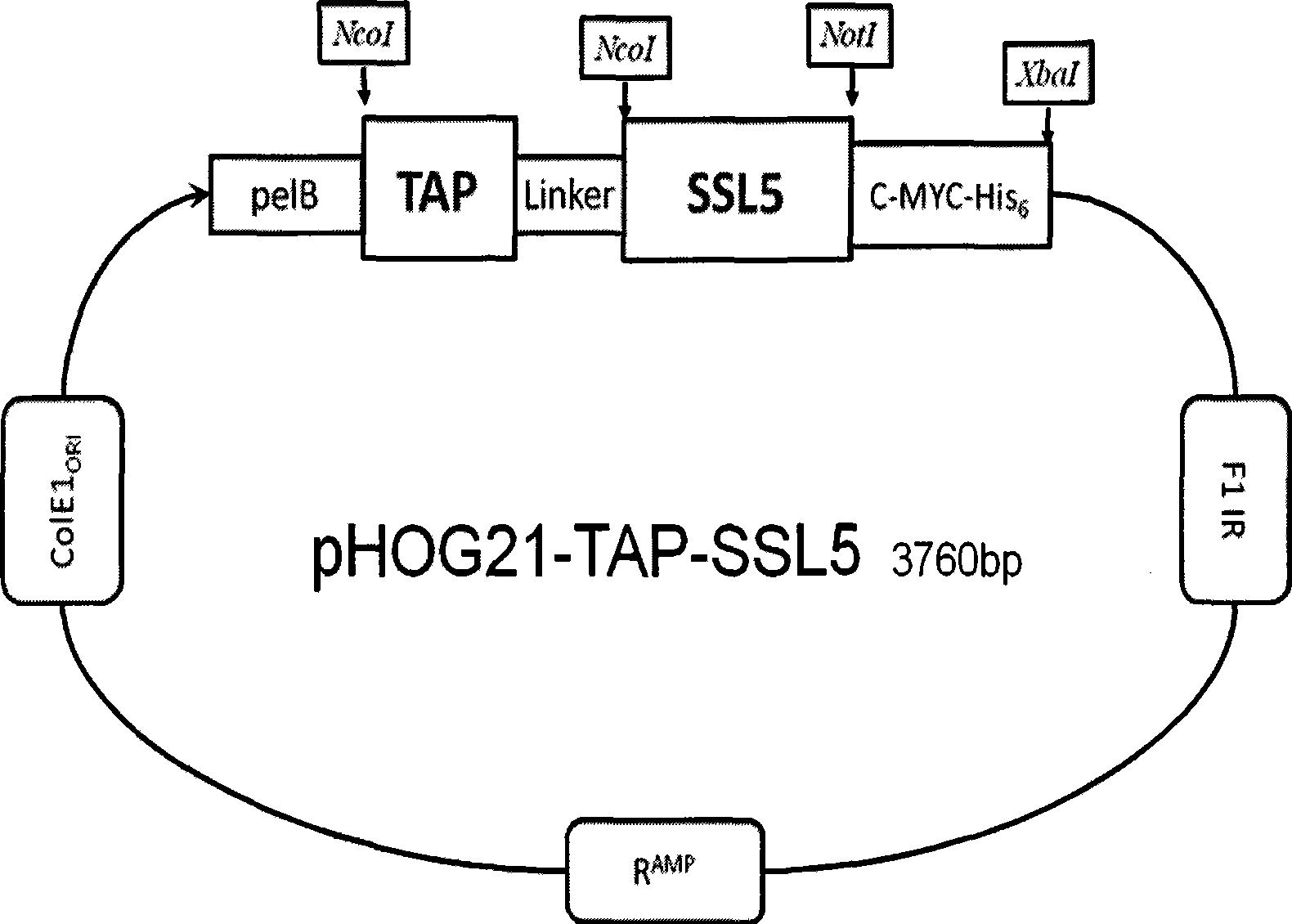
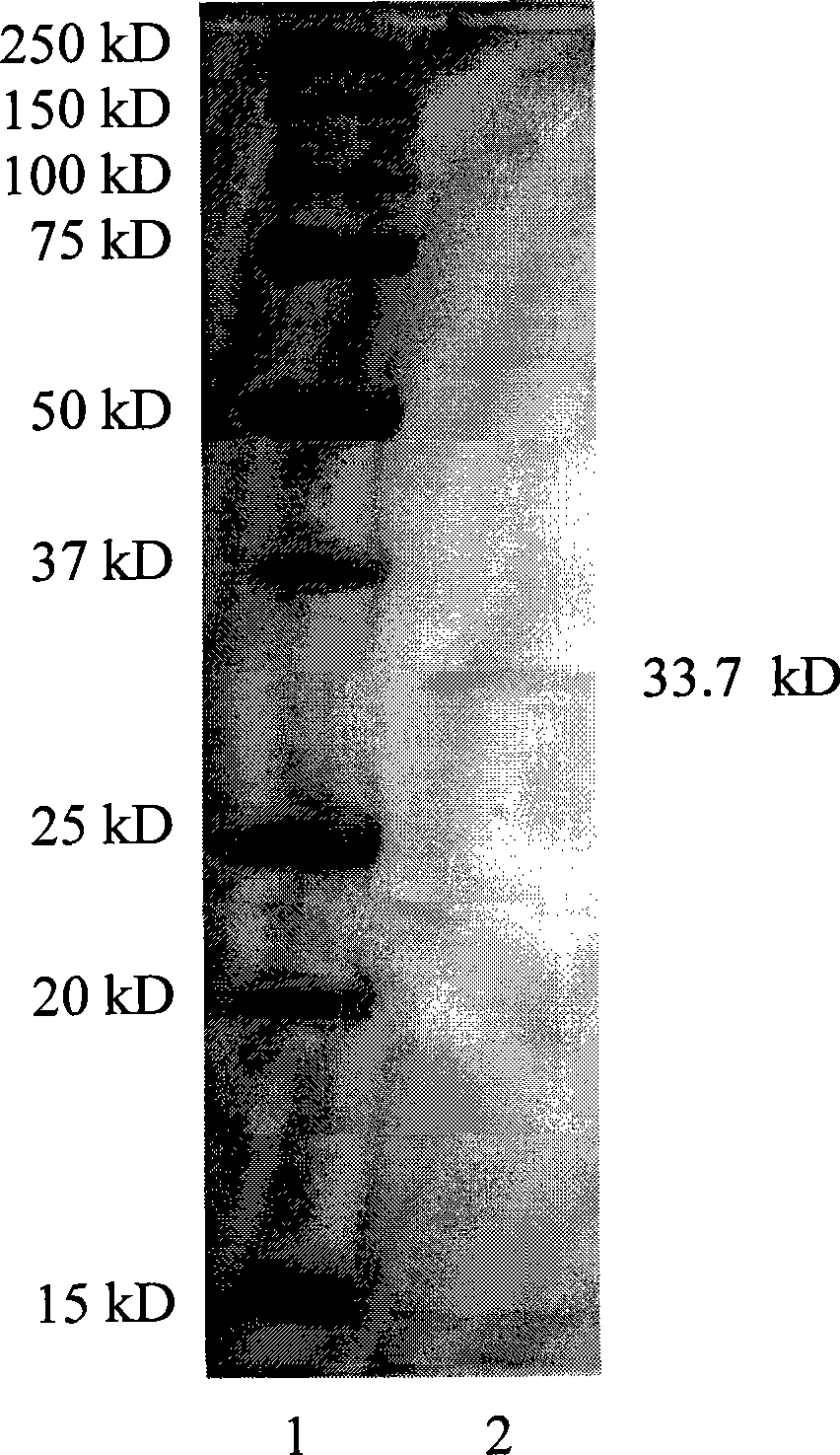
[0029] The amino acid sequence of the TAP-SSL5 fusion protein of the present invention includes a first region having at least 85% identity to the sequence shown in SEQ ID No.1 and a first region having at least 85% identity to the sequence shown in SEQ ID No.2. The second region; wherein: the sequence shown in SEQ ID No.1 is the amino acid sequence of TAP composed of 60 amino acids, and the sequence shown in SEQ ID No.2 is the amino acid sequence of SSL5 composed of 204 amino acids; in the art, Proteins with at least 85% sequence identity generally do not alter the biological activity of the native protein; therefore, the TAP-SSL5 fusion protein of the present invention includes a first region consisting of TAP or a TAP-derived protein and a first region derived from SSL5 or SSL5. These derivative proteins include, but are not limited to: (1) proteins with one or more amino acid residues substituted, deleted or / and inserted, and the substituted or inserted amino acid residues ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com