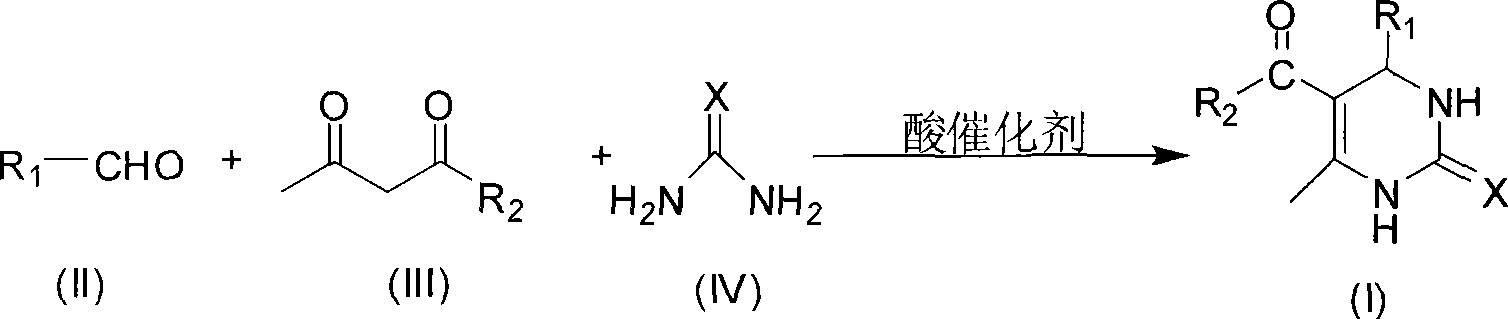
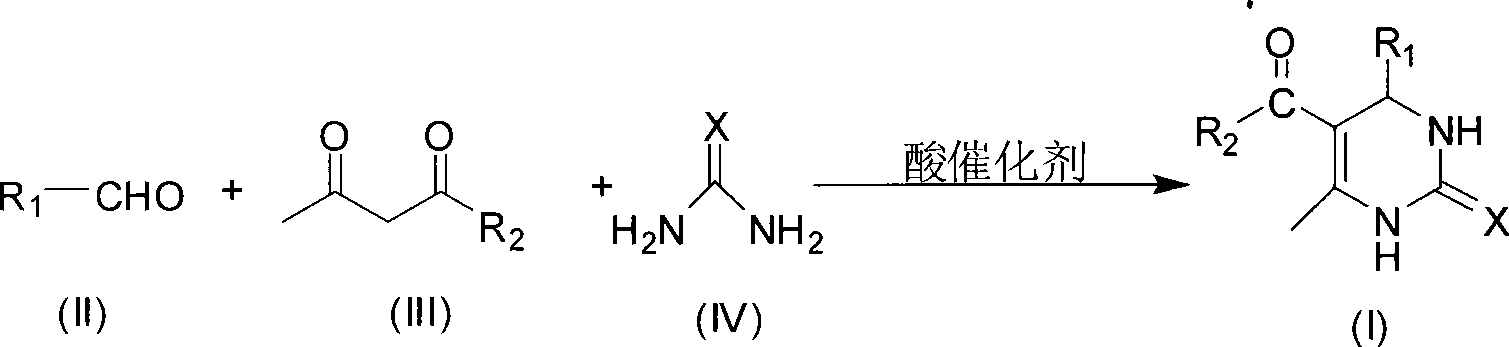
Room temperature solvent-free synthesis of 3,4-dihydropyrimidine-2-ketone
A synthesis method, dihydropyrimidine technology, applied in 3 fields, can solve the problems of not meeting the requirements of green chemistry, long reaction time, low yield, etc., and achieve the effect of low production cost, simple operation and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 100mL round bottom flask, add 21.22g (0.2mol) benzaldehyde, 26.03g (0.2mol) ethyl acetoacetate, 18.02g (0.3mol) urea, 2.88g (0.015mol) citric acid, mix well, and stir at room temperature for 1 After 1 hour, filter with suction, wash with ice water, wash with a small amount of 40% ethanol-water solution, and then wash with water, dry and weigh the solid to obtain 49.94 g of the product, with a yield of 96.0%.
Embodiment 2
[0024] In a 100mL round bottom flask, add 21.22g (0.2mol) benzaldehyde, 20.03g (0.2mol) acetylacetone, 18.02g (0.3mol) urea, 3.84g (0.02mol) citric acid, mix well, and stir at room temperature for 1.5 hours, Suction filtration, washing with ice water, washing with a small amount of 40% ethanol-water solution, washing with water, and weighing the solid to obtain 44.89 g of the product, with a yield of 97.6%.
Embodiment 3
[0026] In a 100mL round bottom flask, add 21.22g (0.2mol) benzaldehyde, 26.03g (0.2mol) ethyl acetoacetate, 18.02g (0.3mol) urea, 1.81g (0.02mol) oxalic acid, mix well, and stir at room temperature for 1.5 hours , filtered with suction, washed with ice water, washed with a small amount of 40% ethanol-water solution, washed with water, dried and weighed the solid to obtain 51.02 g of the product, with a yield of 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com