A kind of rare earth ionic liquid and its preparation method and its application in detecting ferric ion
A technology of rare earth ions and ferric iron, applied in chemical instruments and methods, measuring devices, instruments, etc., can solve the problems of endangering inspectors' health, inconvenient production and use, harmful environment, etc., and achieves convenient and fast detection process and high detection effect Good, short synthetic cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1 rare earth ionic liquid
[0037] 1) Synthesis of intermediate 1-butyl-3-methylimidazolium bromide
[0038] N-methylimidazole and butane bromide were distilled under reduced pressure separately for use.
[0039] Heating with a silicone oil bath to raise the temperature of N-methylimidazole to 50°C, and then adding bromobutane dropwise into the three-necked flask containing N-methylimidazole with a constant pressure dropping funnel, the molar ratio of the two is 1:1.1, after the dropwise addition, react at 50°C-60°C under reflux for about 48h. Filter and recrystallize with a mixture of acetonitrile and ethyl acetate (the volume ratio of acetonitrile and ethyl acetate is 1:2) with a total volume of 30 mL to obtain the intermediate 1-butyl-3-methylimidazolium bromide, the reaction formula is as follows :
[0040]
[0041] Intermediate 1-Butyl-3-methylimidazolium Bromide
[0042] 2) Synthesis of intermediate 1-butyl-3-methylimidazole nitr...
Embodiment 2
[0049] Example 2 Application of Rare Earth Ionic Liquids as Fluorescent Probes in Detection of Ferric Ions
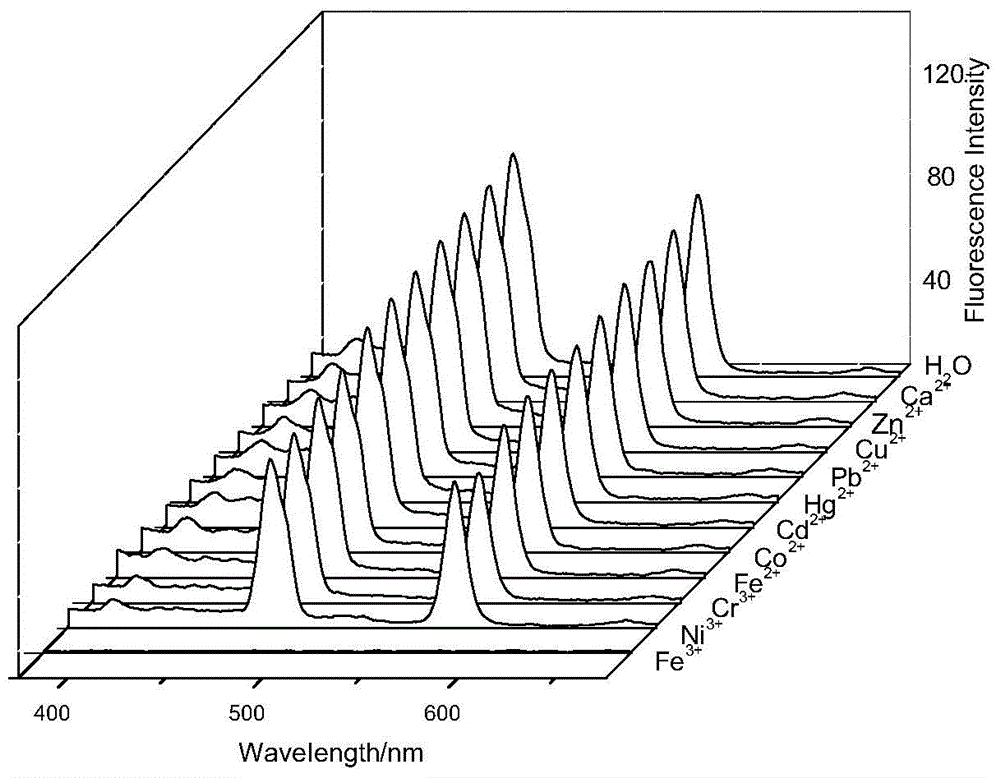
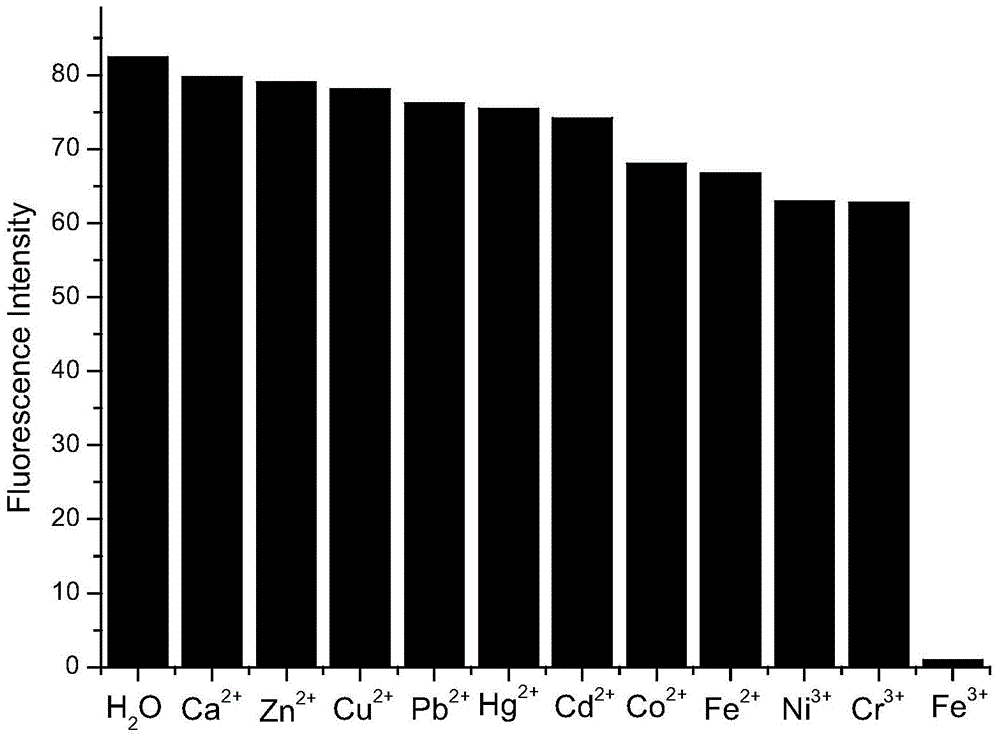
[0050] Accurately add 2.5mL standard detection solution (aqueous solution of 1mol / L rare earth ionic liquid) to several cuvettes, and then add 10μL aqueous solution of 1mol / L metal cation (Zn 2+ , Pb 2+ 、Cd 2+ , Hg 2+ 、Co 2+ 、Ni 3+ 、Cr 3+ , Ca 2+ , Fe 2+ , Fe 3+ 、Cu 2+ ), so that the final concentration of metal cations is 4×10 -3 mol / L, respectively measure its fluorescence spectrum at 325nm excitation wavelength, the results are as follows figure 1 shown. Draw a histogram of the fluorescence intensity of different metal cations at 480nm, such as figure 2 shown.
[0051] From figure 1 and figure 2 It can be seen from the figure that at the excitation wavelength of 325nm, the addition of other heavy metal cations does not cause a significant change in the fluorescence intensity, only the addition of Fe 3+ After that, the fluorescence intensity of the f...
Embodiment 3
[0052] Example 3 Rare earth ionic liquid is used as a fluorescent probe to determine the detection limit of ferric ions
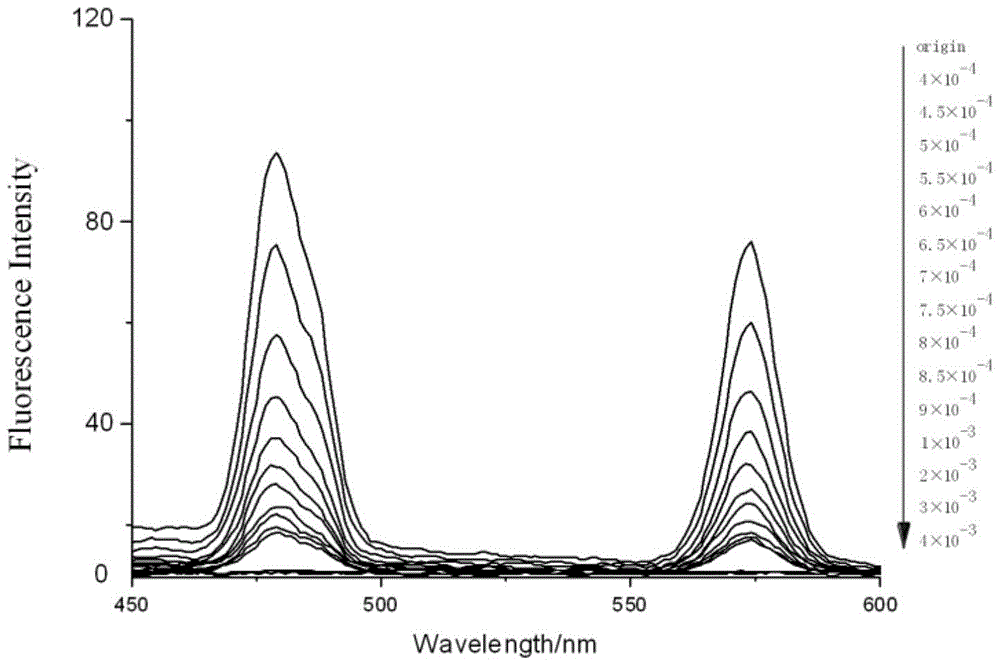
[0053] Accurately add 2.5mL of standard detection solution (aqueous solution of 1mol / L rare earth ionic liquid) to several cuvettes, and then add 10μL of different concentrations of Fe with a pipette gun 3+ Aqueous solutions (concentrations from low to high are 4×10 -4 , 4.5×10 -4 , 5×10 -4 , 5.5×10 -4 , 6×10 -4 , 6.5×10 -4 , 7×10 -4 , 7.5×10 -4 , 8×10 -4 , 8.5×10 -4 , 9×10 -4 、10 -3 , 2×10 -3 , 3×10 -3 , 4×10 -3 mol / L), respectively measure its fluorescence spectrum at 325nm excitation wavelength, such as image 3 shown. Draw a histogram of the fluorescence intensity at 480nm of different concentrations of ferric ions, such as Figure 4 shown.
[0054] From image 3 and Figure 4 It can be seen that as the concentration of iron ions increases, the fluorescence intensity of the fluorescent probe decreases gradually until it is quenched. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com