Process for production of steroids
A compound, cholestanoic acid technology, applied in the field of preparation of steroids, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
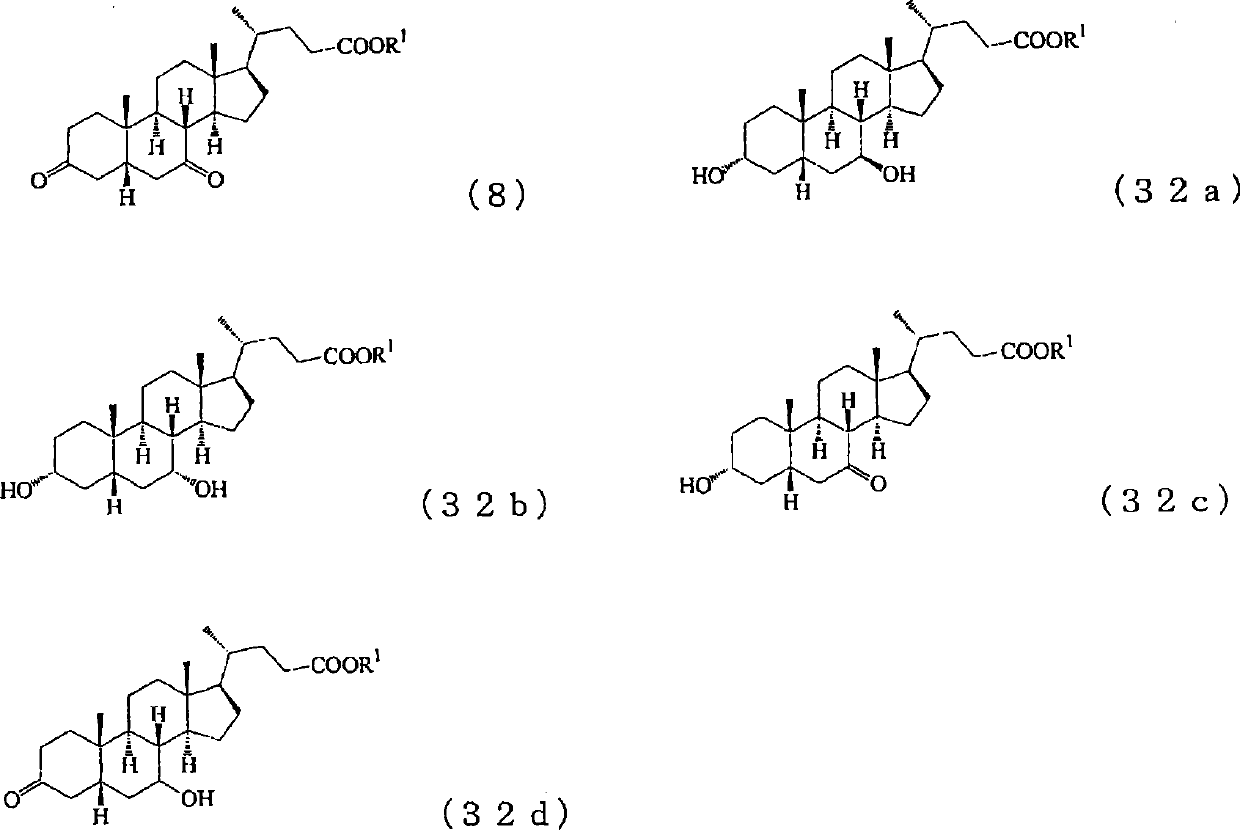
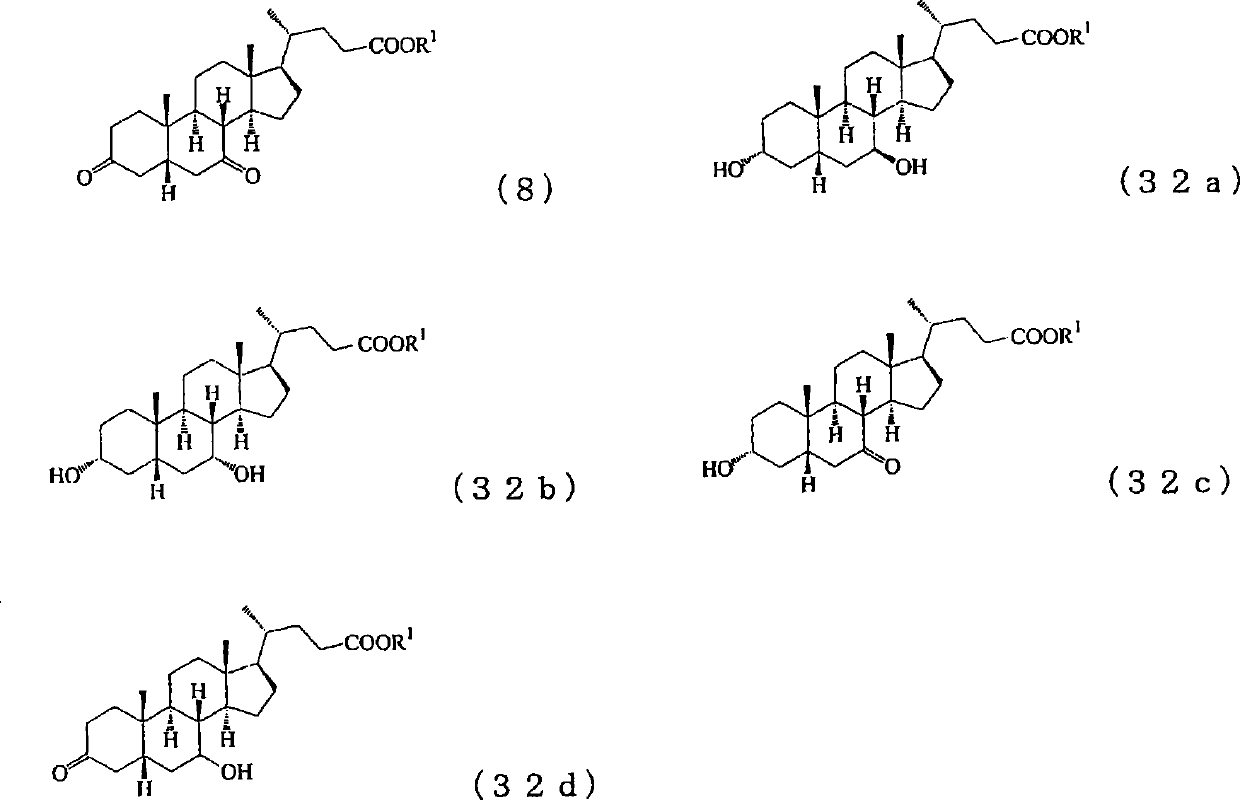
[0307] The cholesta-5,7,24-trien-3β-ol used as a raw material in the production method of the present invention itself is a known substance. For example, it can be produced by metabolically engineering a fungus that produces ergosterol via zymosterol to create a mutant strain, culturing the mutant strain to obtain a culture, and collecting it from the culture. For details of the production method, for example, methods described in JP-A-5-192184 and JP-A-2004-141125 can be referred to.
[0308] Preparation of cholesta-4,7,24-triene-3 represented by the following formula (3) from cholesta-5,7,24-trien-3β-ol represented by the following formula (2) - Keto steps
[0309]
[0310] As can be seen from the above reaction formula, step 1 of the present invention is to simultaneously carry out the oxidation of the 3-position hydroxyl and the 5-position double bond of cholesta-5,7,24-triene-3β-alcohol (hereinafter sometimes referred to as "compound 2") A step toward the isomerizat...
Embodiment 1
[0518]
[0519] 4.38g (11.47mmol) of cholesta-5,7,24-triene-3β-alcohol (compound 2) and 11.24g (114.66mmol) of cyclohexanone were dissolved in 44ml of toluene, and the reduction was repeated several times at room temperature. After pressure degassing and nitrogen substitution, 1.17 g (5.74 mmol) of aluminum isopropoxide was added at room temperature, and the mixture was stirred at 112° C. for 2 hours under a nitrogen atmosphere. After the reaction, the reaction liquid was cooled to room temperature, 2.3ml of water was added and stirred at room temperature for 1 hour. The precipitated precipitate was filtered, the filtrate was concentrated, and it was separated and purified by silica gel column chromatography to obtain 4.01 g of cholesta-4,7,24-trien-3-one (compound 3). The yield of isolated and purified compound 2 was 92%, and its NMR shift value (δppm) is shown below.
[0520] δ: 0.60(s, 3H, 18-H), 0.96(d, 6.5Hz, 3H, 21-H), 1.18(s, 3H, 19-H), 1.61(s, 3H, 26-H), 1.69 (s, 3...
Embodiment 2
[0522]
[0523] 3.49g (9.18mmol) of cholesta-4,7,24-triene-3-one (compound 3) obtained by the method of Example 1 was dissolved in 70ml of methanol, and several times of vacuum decompression were carried out at room temperature. After gas and nitrogen substitution, 2.53 g (38.40 mmol) of 85% granular potassium hydroxide was added, and the mixture was stirred at 64° C. for 7 hours under a nitrogen atmosphere. After the reaction, the reaction liquid was cooled to room temperature, 2.37 g of acetic acid was added and stirred at room temperature for 0.5 hours. Next, methanol was distilled off under reduced pressure, water was added, and extracted with ethyl acetate, the organic phase was washed with water, dried, concentrated, and separated and purified by silica gel column chromatography to obtain 3.13 g of cholestan-4,7,24- Trien-3-ones (Compound 4). The yield of isolated and purified compound 3 was 90%, and its NMR shift value (δppm) is shown below.
[0524] δ: 0.76(s, 3H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com