Preparation method of arginine aspirin and powder and injection preparation thereof
A technology of arginine aspirin and aspirin, which is applied in the field of medicine, can solve problems such as long time, high cost, and low efficiency, and achieve the effect of saving time and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 arginine aspirin
[0022] (1) Dissolve 209.1g of arginine in water for injection, add 180.2g of aspirin, stir evenly, raise the temperature to 80°C, keep stirring for 4h, make it fully react, and obtain a clear solution; filter, add an appropriate amount of chloroform, and stir for 40min , stored at a low temperature of 2-8°C for 10 hours, precipitated out, filtered, washed with an appropriate amount of ethanol, and vacuum-dried at 50°C for 6 hours to obtain 303.3 g of arginine aspirin crude product;
[0023] (2) Put 303.3g of arginine aspirin crude product and 2420ml of 80% ethanol solution into the reaction flask, heat to 70°C, stir to make all the solids dissolve, add 2.72g of gac (equivalent to adding 0.1g gac to 1ml solution), stir for 30min, Filtrate, add an appropriate amount of absolute ethanol to the filtrate, place it at 2-8°C for low-temperature crystallization, filter, wash the filter cake with ethanol, and dry it in vacuum at...
Embodiment 2
[0024] The preparation of embodiment 2 arginine aspirin
[0025] (1) Dissolve 418.3g of arginine in water for injection, add 360.5g of aspirin, stir evenly, raise the temperature to 60°C, keep stirring for 2h, make it fully react, and obtain a clear solution; filter, add an appropriate amount of chloroform, and stir for 20min , stored at 2-8°C for 20 hours at a low temperature, precipitated, filtered, washed with an appropriate amount of ethanol, and vacuum-dried at 70°C for 4 hours to obtain 597.2 g of arginine aspirin crude product;
[0026] (2) Put 597.2g of arginine aspirin crude product and 4800ml of 80% ethanol solution into the reaction flask, heat to 60°C, stir to make all the solids dissolve, add 24.33g of activated carbon (equivalent to 1ml solution plus 0.5g of activated carbon), stir for 10min, Filtrate, add an appropriate amount of absolute ethanol to the filtrate, place it at 2-8°C for low-temperature crystallization, filter, wash the filter cake with ethanol, an...
Embodiment 3
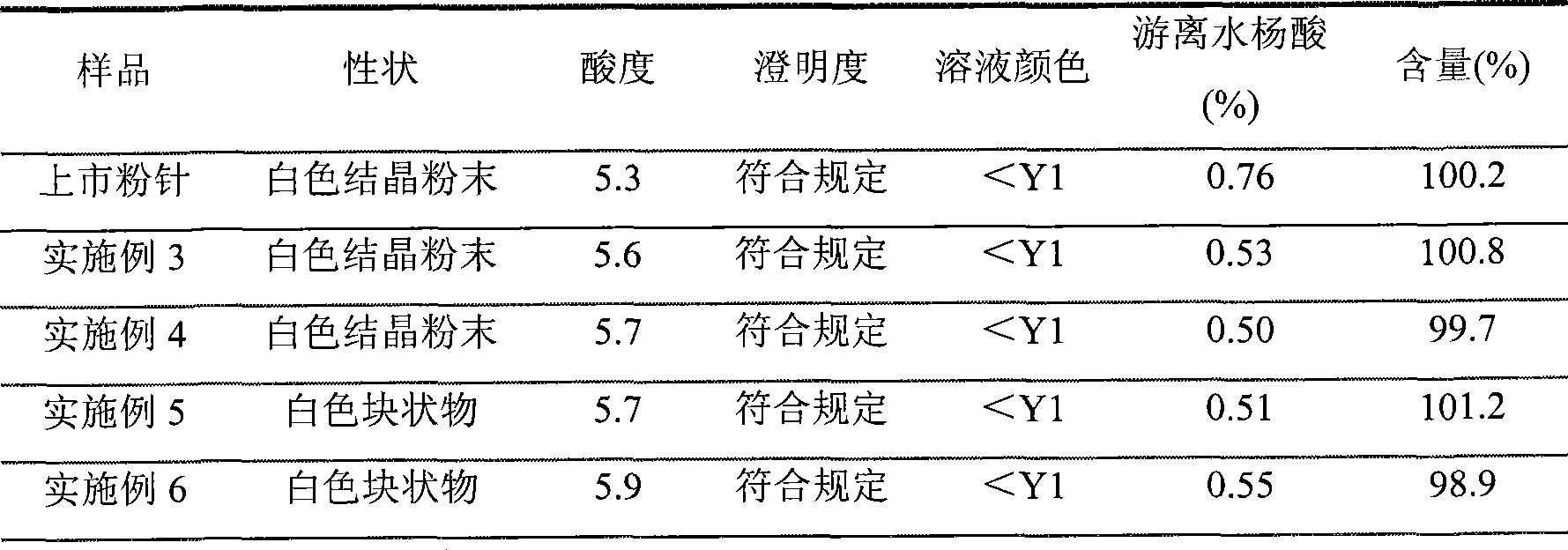
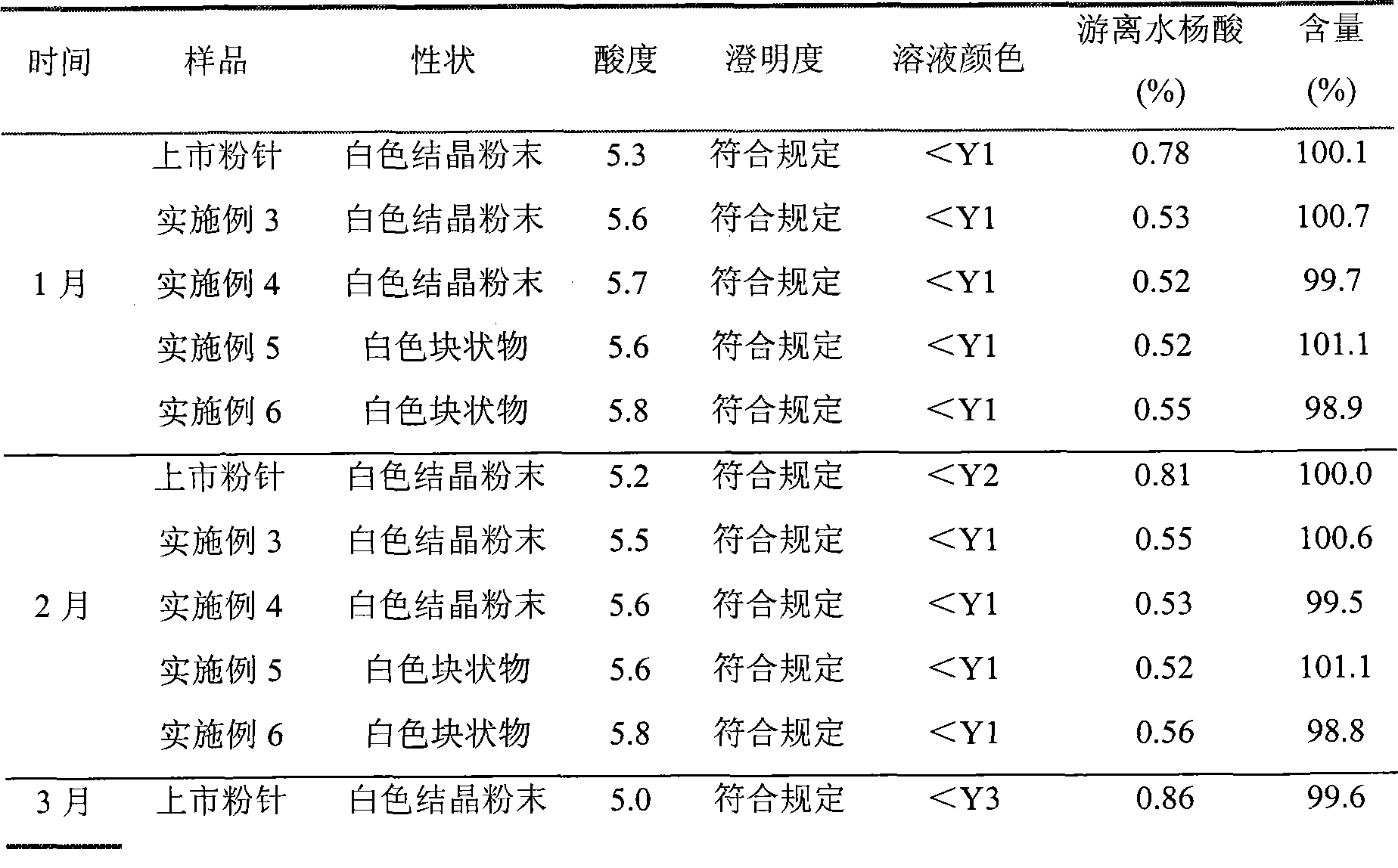
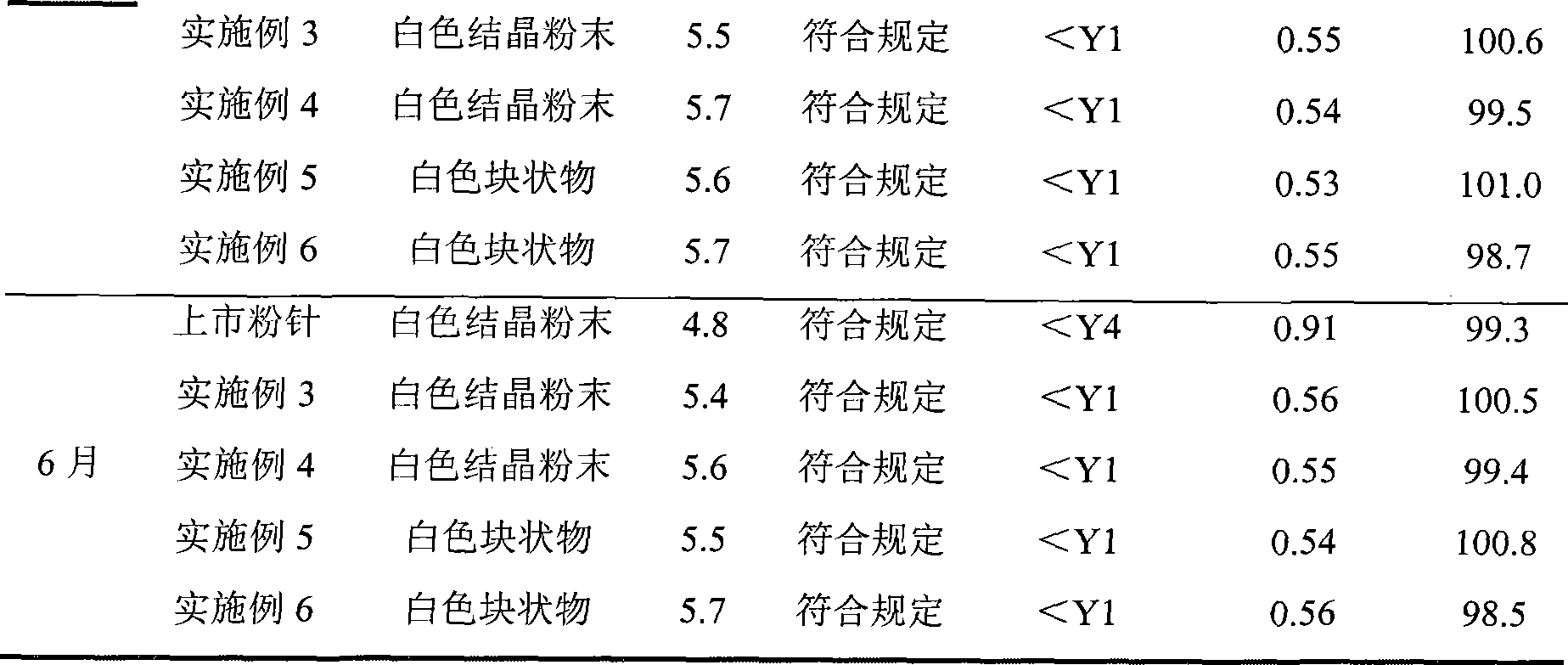
[0028] The refined product of aspirin arginine prepared in Example 1 was subpackaged under 100-grade clean conditions in a sterile room, and each bottle contained 0.5 g of aspirin arginine to obtain aspirin arginine powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com