Process for the production of preformed conjugates of albumin and a therapeutic agent
A technology for conjugates and albumin, which is applied in the field of preparation of preformed conjugates of albumin and therapeutic agents, and can solve problems such as the limitation of therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] 6.1 Example 1: Purification of recombinant albumin expressed by Pichia pastoris
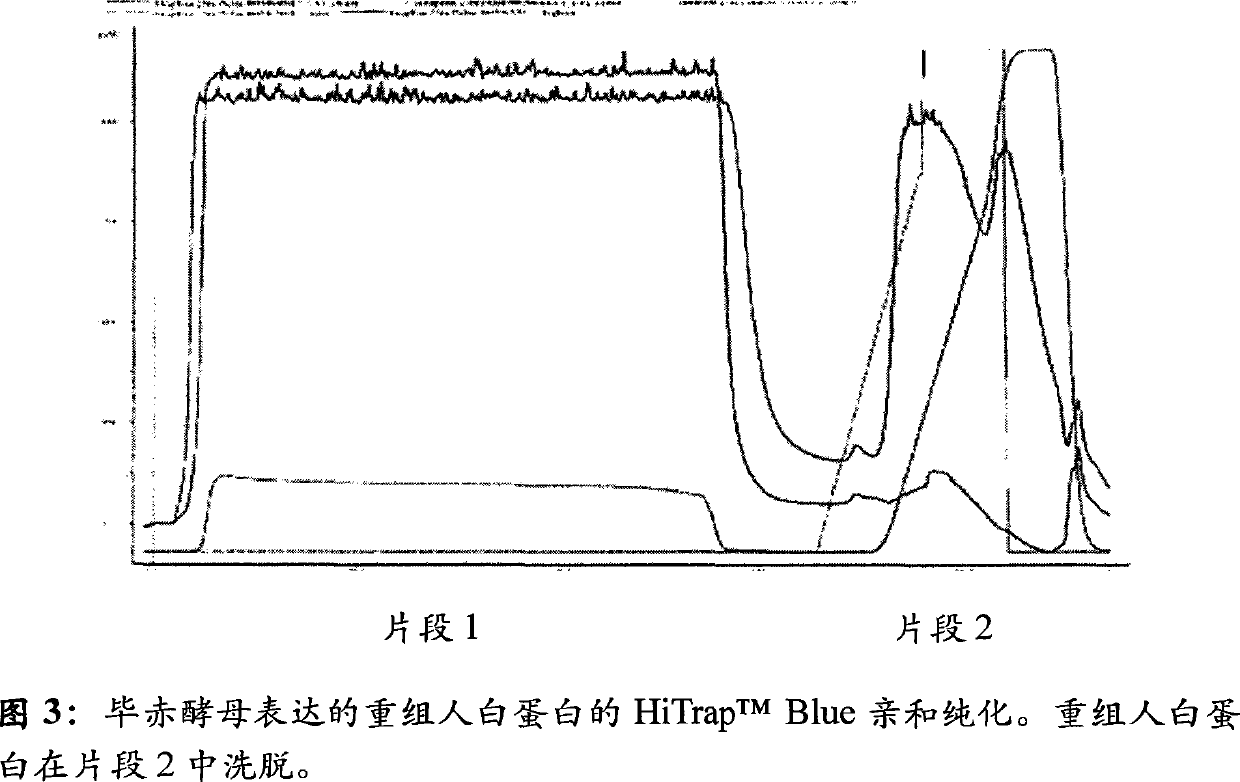
[0263] This example illustrates the purification of recombinant albumin expressed by Pichia pastoris by different chromatographic methods. Recombinant albumin was expressed using the Pichia expression kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
[0264] 6.1.1 DEAE Sepharose: Weak Anion Exchange Chromatography
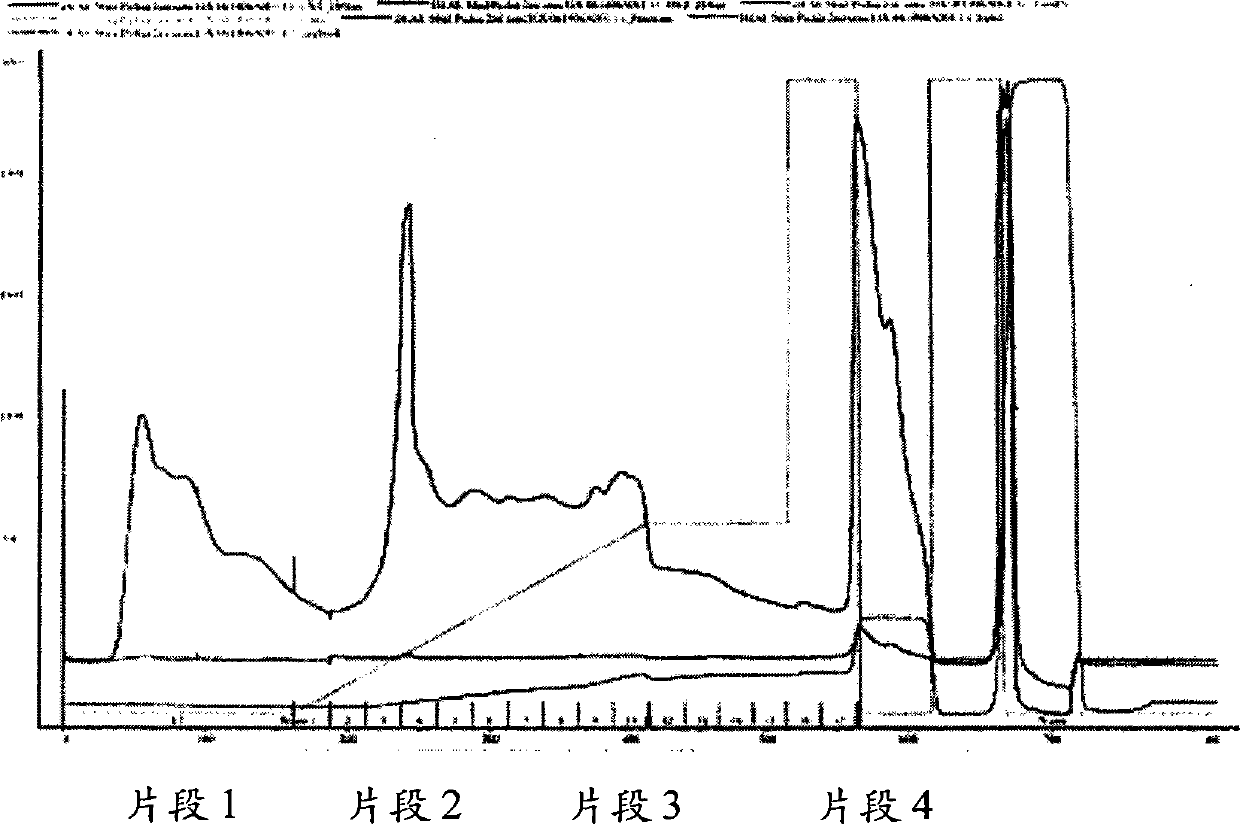
[0265] Purification of Pichia-expressed recombinant albumin was performed on a DEAE Sepharose column equilibrated with pH 7.0, 10 mM phosphate buffer. The following increasing salt gradients (50 ml column volume, 2 ml / min flow rate) were used: 66 mM sodium phosphate over 5 column volumes; 66 mM sodium phosphate over 2 column volumes; 200 mM sodium phosphate over 0 column volumes; Regenerated in column volume of 200 mM sodium phosphate; pH 8.0, 20 mM Tris-HCl buffer and 2 M NaCl. The purified albumin fragments in Figure 1 were eluted in fractions f...
Embodiment 2
[0272] 6.2 Example 2: Purification of recombinant albumin after mercaptoalbumin enrichment
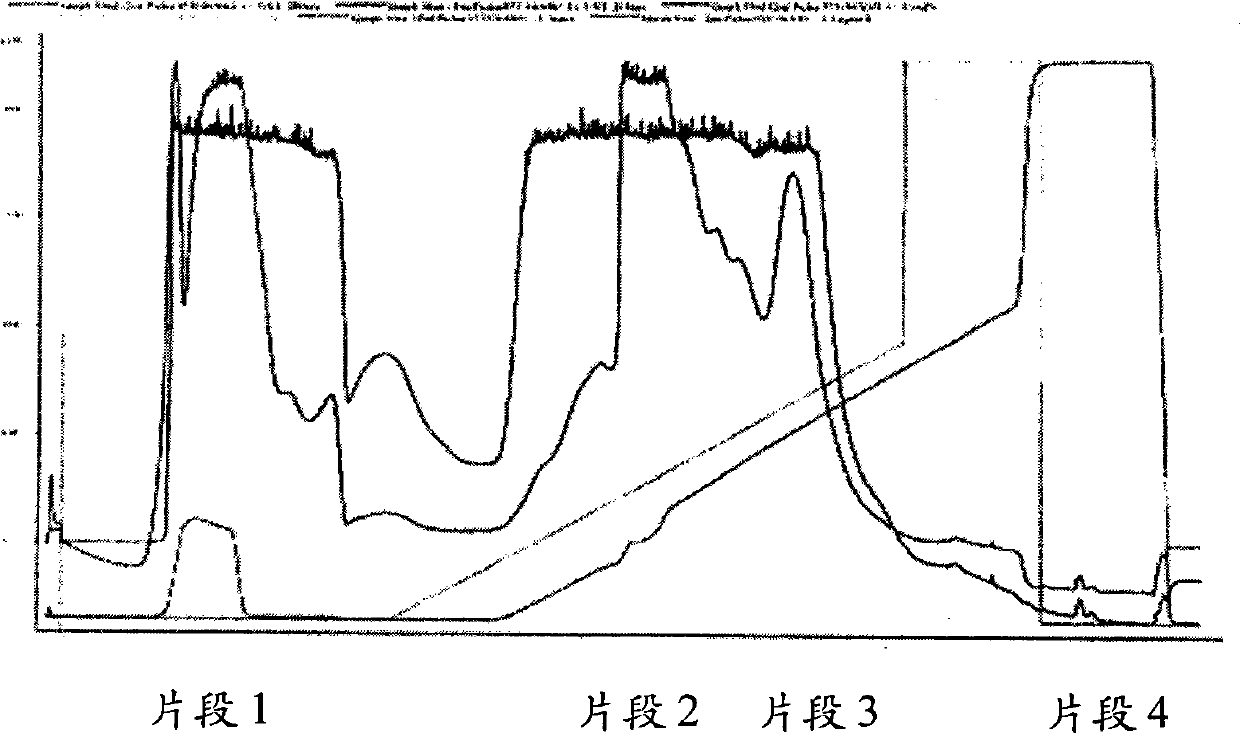
[0273] This example illustrates the purification of Pichia pastoris expressed and mercaptoalbumin-enriched recombinant albumin using phenyl sepharose hydrophobic interaction chromatography. Recombinant albumin (0.2% final concentration) was treated with 74 mM thioglycolic acid in 250 mM Tris-acetic acid buffer for 20 hours at 4°C. Purified with pH 7.0, 20 mM sodium phosphate, 5 mM sodium caprylate and 750 mM (NH 4 ) 2 SO 4 Performed on a phenyl sepharose column equilibrated in medium. A decreasing salt gradient (5 ml column volume, 5 ml / min flow rate) was used as follows: 20 mM sodium phosphate, 5 mM sodium caprylate over 2 column volumes; washing with water over 1 column volume; 20% over 1 column volume ethanol; and more than 1 column volume of water. In Figure 5, the purified albumin fraction was in the range of 750 to 0 M (NH 4 ) 2 SO 4 Elution in a decreasing gradient. F2 ...
Embodiment 3
[0274] 6.3 Example 3: Purification of recombinant albumin after deglycosylation
[0275] This example illustrates the deglycosylation of human serum albumin by affinity chromatography using amino-phenylboronic acid and concanavalin A as ligands. Chromatography was performed in an AKTA purification unit (Amersham Biosciences, Uppsala, Sweden).
[0276] 6.3.1 Amino-phenylboronic acid chromatography on agarose
[0277] Amino-phenylboronic acid resin including agarose (Sigma, St. Louis, MO) was used pH 8.5, 4 column volumes of 0.25M ammonium acetate, 0.05MgCl 2 (0.5ml / min flow rate) rinse and equilibrate. A 25% solution of human serum albumin (Cortex Biochem, San Leandro, CA) was diluted 1:2 in equilibration buffer and loaded onto the column. The flow through (F3) was collected and the column was washed with 4 column volumes of equilibration buffer. Elution was performed with 3 column volumes of pH 8.5, 0.1M Tris and 0.2M sorbitol and collected in F2. F3 and F2 were concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com