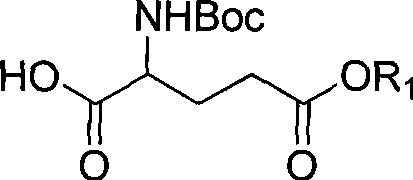
Method for preparing stereoselectivity of complete protected 2-amido-6-hydroxyl-4-methyl-8-carbonyl capric acid
A technology of stereoselectivity and carbonyl decanoic acid, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of cyanide reactions. Selectivity and other issues, to achieve a good effect of stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
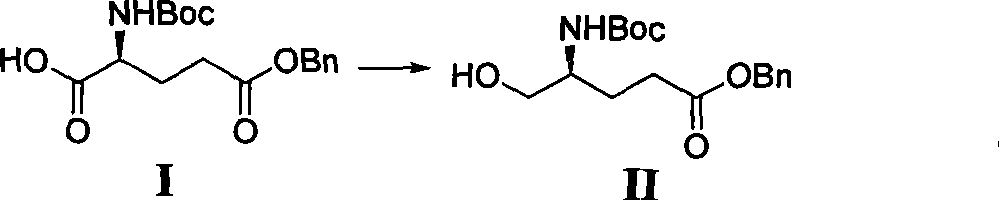
[0008] The preparation method of the fully protected 2-amino-6-hydroxyl-4-methyl-8-carbonyldecanoic acid of the present invention can be obtained by (2S, 4S, 6S)-2-(tert-butoxycarbonyl) amino-6- The preparation of benzyloxy-4-methyl-8-carbonyldecanoic acid methyl ester is specifically described, including the following steps: 1, glutamic acid derivatives are introduced into the Evans chiral prosthetic group, which is divided into four steps and carried out, and the specific steps are as follows Shown:
[0009] In the first step, glutamate is reduced to glutamine:
[0010]
[0011] 0.33mol of N-tert-butoxycarbonylglutamic acid-γ-benzyl ester (compound I in the figure) was dissolved in 1600ml of anhydrous tetrahydrofuran and cooled with an ice-salt bath. Add 56ml of triethylamine, then slowly drop into 35ml of ethyl chloroformate. After 30 min, the ice-salt bath was changed to an ice bath, and after stirring for 30 min, a solution of sodium borohydride (37 g) in methanol (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com