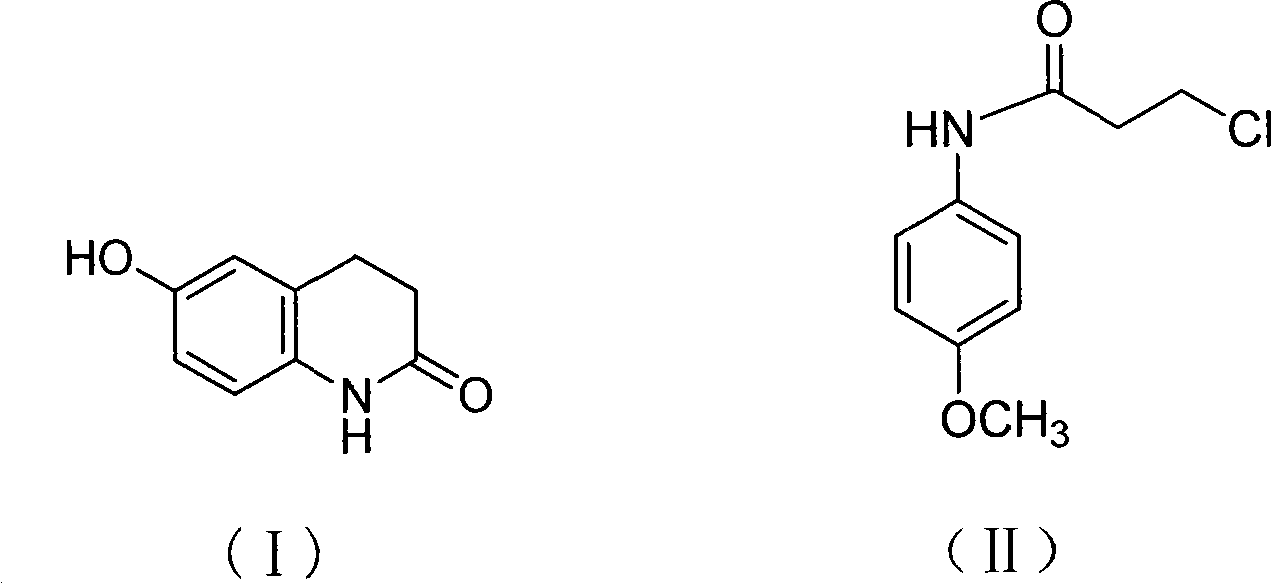
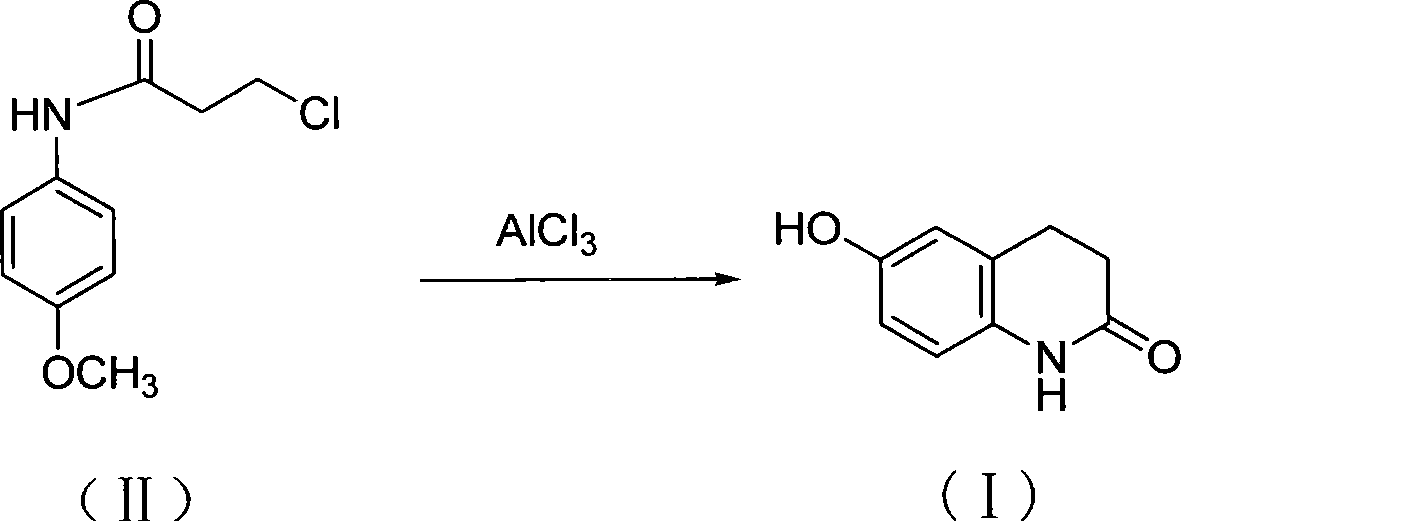
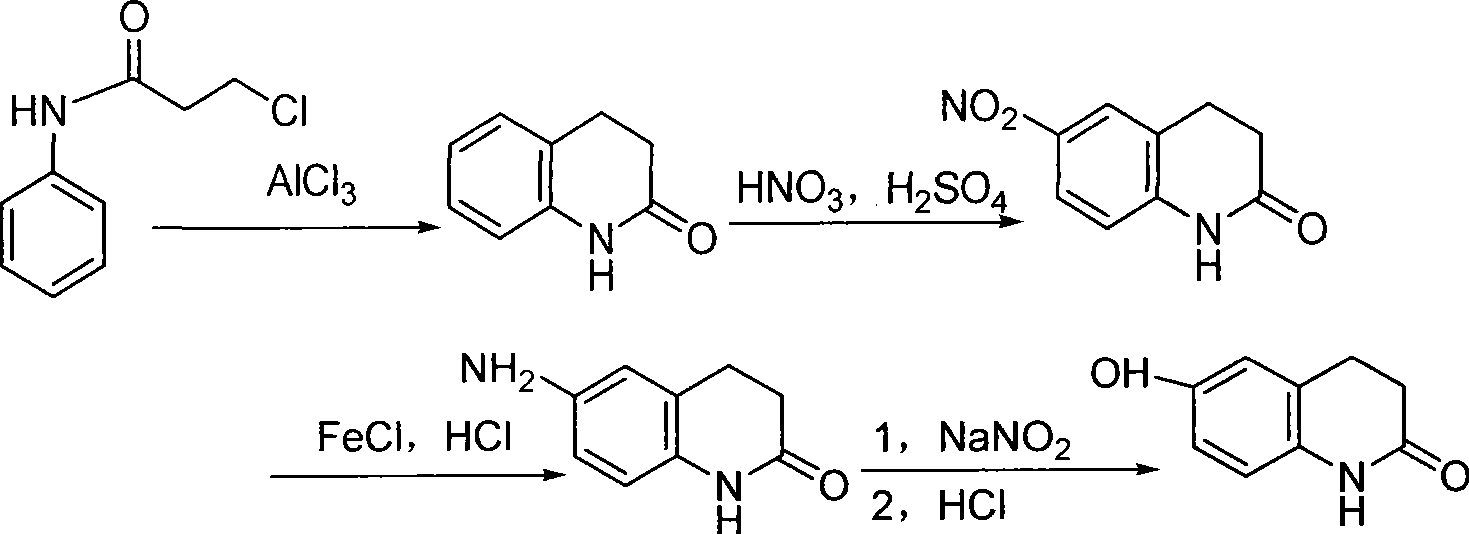
Method for preparing 6-hydroxyl-3,4dihydro-2(1H)-quinolinone
A technology of quinolinone and dihydro, which is applied in the field of preparation of 6-hydroxy-3,4dihydro-2(1H)-quinolinone, can solve problems such as high cost, high viscosity of the system, and environmental pollution, and achieve Ease of control, improvement of product yield and quality, and avoidance of the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 10g (0.05mol) of the compound of formula II, 25g (0.19mol) of aluminum trichloride, and 7.10g (0.12mol) of sodium chloride into a 250mL three-neck flask, mix well, stir mechanically, heat up to 160-170°C and keep it warm for 2 hours , cooled to 60°C, added a mixture of 50mL of concentrated hydrochloric acid and 100g of crushed ice, stirred slowly, a brick red solid precipitated, cooled to room temperature, filtered with suction, washed with pure water until neutral to obtain a crude product. The above crude product was recrystallized with ethanol and decolorized with activated carbon to obtain 6.2 g of off-white crystals of the compound of formula I with a yield of 81.2%.
Embodiment 2
[0021] Add 250g (1.17mol) of the compound of formula II, 624g (4.68mol) of aluminum trichloride, and 177g (3.04mol) of sodium chloride into a 5L three-neck flask, mix well, stir mechanically, heat up to 170-180°C and keep it warm for 2 hours. Cool down to 140°C, slowly pour the system into a mixture of 1250mL concentrated hydrochloric acid and 2500g crushed ice, stir slowly, a brick-red solid precipitates, cool to room temperature, filter with suction, wash with pure water until neutral, and obtain a crude product. The above crude product was recrystallized with ethanol and decolorized with activated carbon to obtain 168.0 g of off-white crystals of the compound of formula I with a yield of 88.0%.
Embodiment 3
[0023] Add 10g (0.05mol) of the compound of formula II, 25g (0.19mol) of aluminum trichloride, and 8.95g (0.12mol) of potassium chloride into a 250mL three-neck flask, mix well, stir mechanically, heat up to 160-170°C and keep it warm for 2 hours , cooled to 60°C, added a mixture of 50mL of concentrated hydrochloric acid and 100g of crushed ice, stirred slowly, a brick red solid precipitated, cooled to room temperature, filtered with suction, washed with pure water until neutral to obtain a crude product. The above crude product was recrystallized with ethanol and decolorized with activated carbon to obtain 6.1 g of off-white crystals of the compound of formula I with a yield of 79.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com