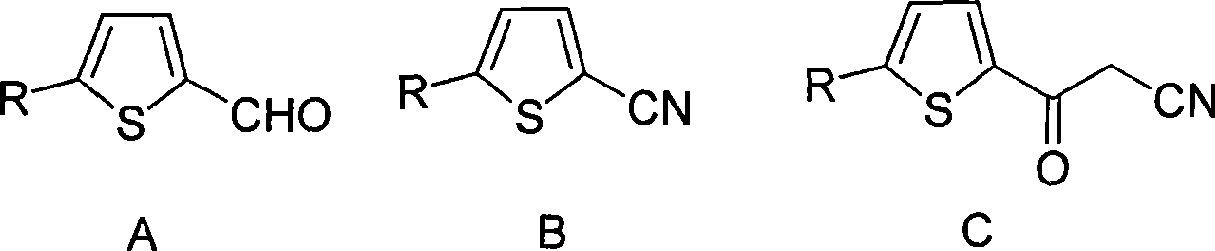
Method for preparing 2-cyanoacet-5-substituted thiophenes compound
A compound, cyanoacetyl technology, applied in the field of synthesis and preparation of organic compounds, can solve problems such as unsatisfactory and difficult product purification, and achieve the effects of convenient operation, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthesis of embodiment 1 5-fluorothiophene carbonitrile
[0018] In the three-necked flask of 250 milliliters, install thermometer, stirrer, join iodine (0.11mol) to 5-fluorothiophene formaldehyde (R=F, 0.1mol), dioxane (100ml), ammoniacal liquor (0.8mol) in the mixture. Stir at 60°C, follow and control the reaction time by HPLC. Then add Na 2 S 2 o 3 Unreacted iodine was removed, cooled to 0°C, stirred for 1 hour, and a large amount of crystals were produced. It was then filtered and dried to obtain the product as a pale yellow solid with a yield of 92%.
Embodiment 2
[0019] The synthesis of embodiment 2 5-chlorothiophene carbonitrile
[0020] In the three-necked bottle of 250 milliliters, install thermometer, stirrer, join iodine (0.1mol) to 5-chlorothiophene formaldehyde (R=Cl, 0.1mol), ether (80ml), in the ammoniacal liquor (1.0mol) mixture. Stir at 40°C, follow and control the reaction time by HPLC. Then add Na 2 S 2 o 3 Unreacted iodine was removed, cooled to 0°C, stirred for 1 hour, and a large amount of crystals were produced. It was then filtered and dried to obtain the product as a pale yellow solid with a yield of 93%.
Embodiment 3
[0021] The synthesis of embodiment 3 5-bromothiophene carbonitrile
[0022] In the 250 milliliter three-necked bottle, install thermometer, stirrer, join iodine (0.4mol) to 5-bromothiophene formaldehyde (R=Br, 0.1mol), n-butyl ether (100ml), ammoniacal liquor (1.2mol) in the mixture. Stir at 20°C, follow and control the reaction time by HPLC. Then add Na 2 S 2 o 3 Unreacted iodine was removed, cooled to 0°C, stirred for 1 hour, and a large amount of crystals were produced. It was then filtered and dried to obtain the product as a pale yellow solid with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com