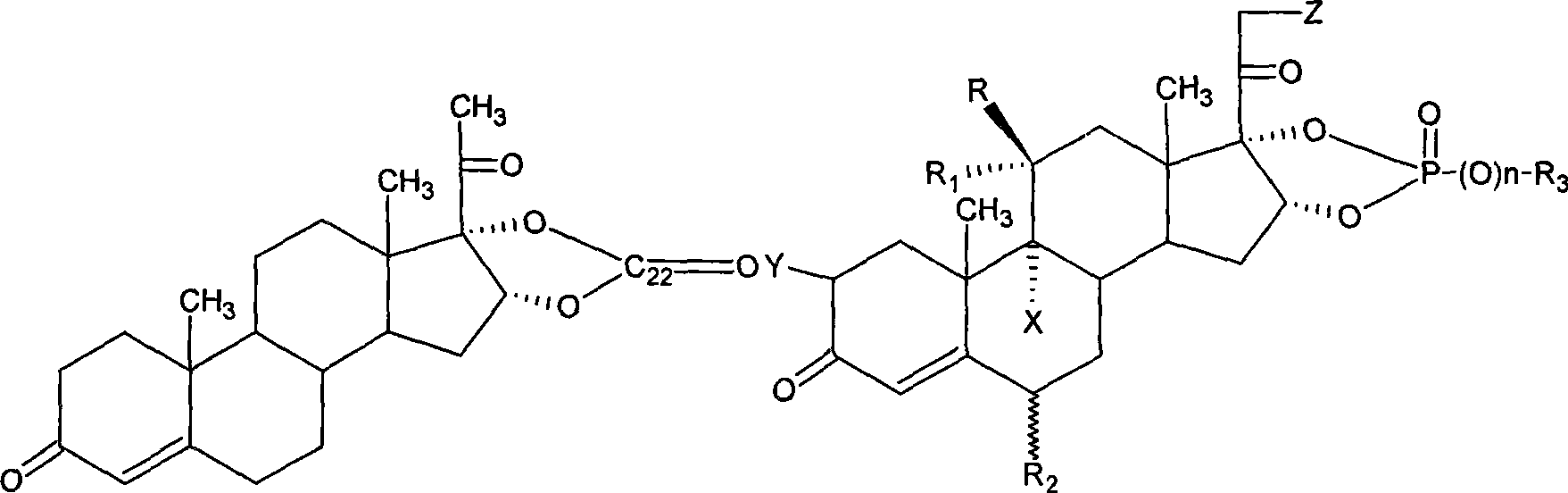
Novel nitrogen-containing medicament with anti-inflammatory activity
A technology of drugs and compounds, applied in the field of androstane glucocorticoid receptor agonist compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
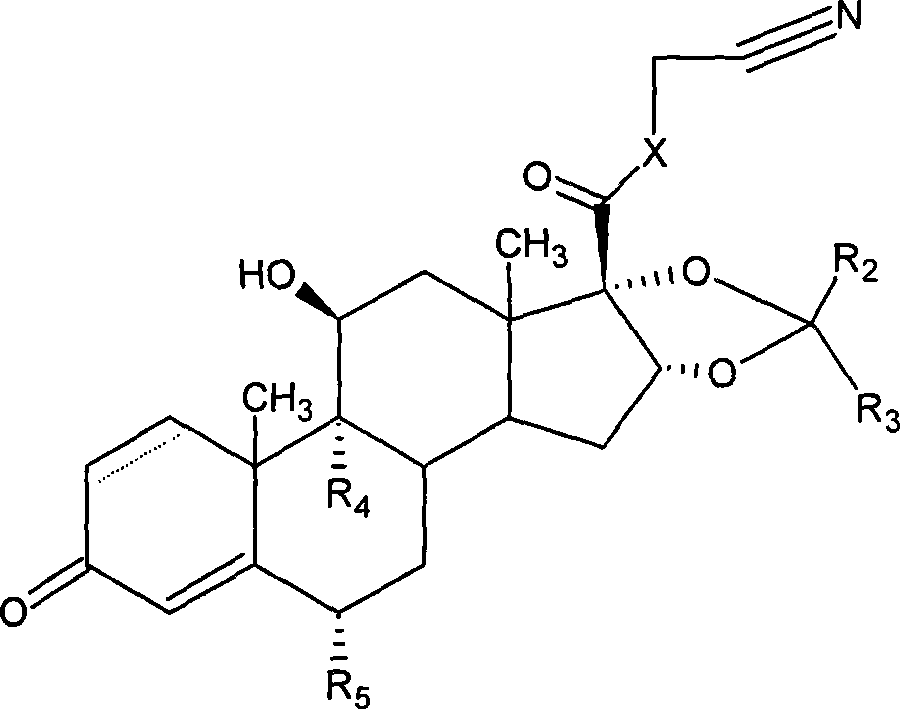
[0210] Example 1: 6α,9α-difluoro-11β-hydroxyl-16α,17α-[(22-Rcyclohexylmethine)dioxy]-3-oxoandrost-1,4-diene-17β- Preparation of nitrile methyl carboxylate
[0211]
[0212] Put 10mmol of 6α, 9α-difluoro-16α, 17α, 11β-trihydroxy-3-oxoandrost-1,4-diene-17β-carboxylic acid nitrile methyl ester, 70% HF50ml into a plastic bottle, ice salt Cool the bath to about -5°C, stir for 10 to 20 minutes, then slowly add 14 mmol cyclohexylformaldehyde dropwise, and keep stirring at -5°C for 1 hour. with 10% NH 4 OH / H 2 About 500ml of O was neutralized and diluted to pH = 7, filtered, and dried to obtain 6α, 9α-difluoro-11β-hydroxyl-16α, 17α-[(22-R,S cyclohexylmethine) dioxygen as a white powdery solid ]-3-oxoandrost-1,4-diene-17β-carboxynitrile methyl ester 8.04mmol, use silica gel 254 to carry out column chromatography, wash with acetone / petroleum ether=4 / 3 as the mobile phase, take the main The point substance is 6α, 9α-difluoro-11β-hydroxyl-16α, 17α-[(22-R cyclohexylmethine) dioxy]-3...
Embodiment 2
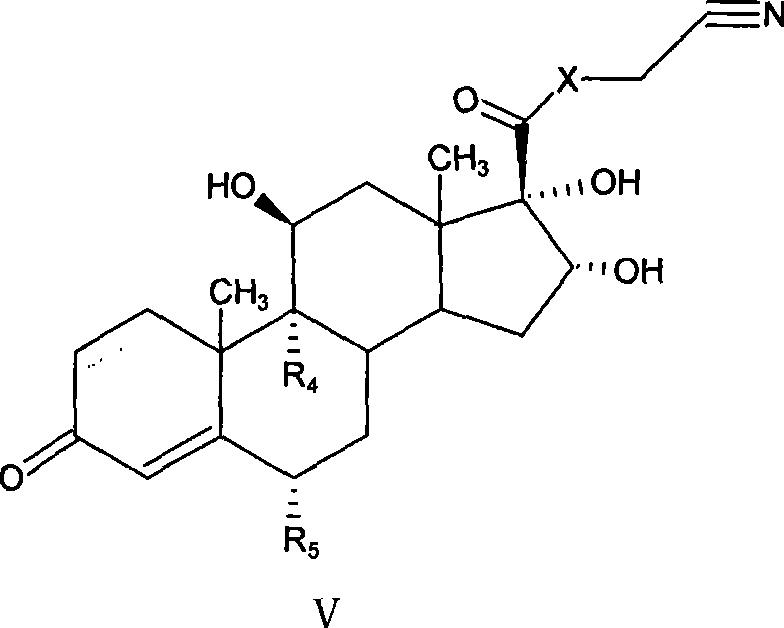
[0218] Example 2: 6α,9α-difluoro-11β-hydroxy-16α,17α-[(22R,S-Propylmethylene)bisoxy-]-3-oxoandrost-1,4-diene- Preparation of 17β-carboxynitrile methyl ester
[0219]
[0220] Dissolve 10mmol of 6α, 9α-difluoro-16α, 17α, 11β-trihydroxy-3-oxoandrost-1,4-diene-17β-carboxynitrile methyl ester in 50ml of dichloromethane under a salt ice bath , 0.7ml 70% HClO 4 , cool down to -5°C, slowly add 14mmol n-butyraldehyde dropwise, keep stirring for 1h, 20% NH 3 / H 2 O was neutralized to neutral, the layers were separated, the organic layer was taken, concentrated under reduced pressure, poured into methanol for recrystallization to obtain 7.43 mmol of the title compound, 22R:S=84:16.
[0221] Elemental analysis calculated value (%): C26H31F2NO6 C, 63.53; H, 6.36; F, 7.73; N, 2.85; O, 19.53
[0222] Elemental analysis found value (%): C 63.76, H 6.41, N 2.79, O 19.38
[0223] 13 C-NMR: Numerical value of carbon from position 1 to position 23
[0224] ( ), 37.1, 13.9, 14.8 (22-CH...
Embodiment 3
[0225] Example 3: 6α,9α-difluoro-11β-hydroxy-16α,17α-[(22R-propylmethylene)bisoxy-]-3-oxoandrost-1,4-diene-17β- Preparation of nitrile methyl carboxylate
[0226]
[0227] 5 mmol of the compound obtained in Example 2 was subjected to column chromatography using silica gel 254, washed with acetone / petroleum ether=4 / 3 as the mobile phase, and 3.43 mmol of the title compound was obtained as the main point substance.
[0228] Two-dimensional nuclear Overhaus effect spectroscopy (NOESY), from which the stereo configuration of protons and substituents was determined. Among them, the 18-methyl H and the 16-position H have NOE effect, and the 22-position H and the 16-position H have an NOE effect, which proves that R2 is H.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com