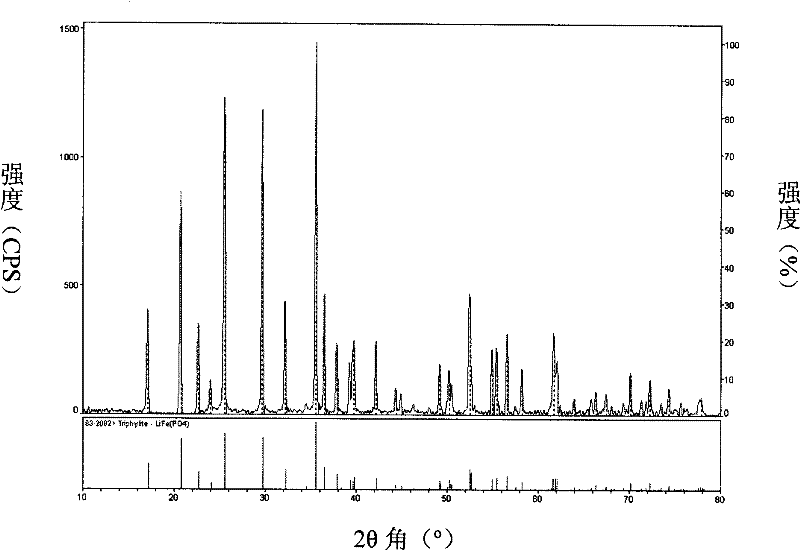
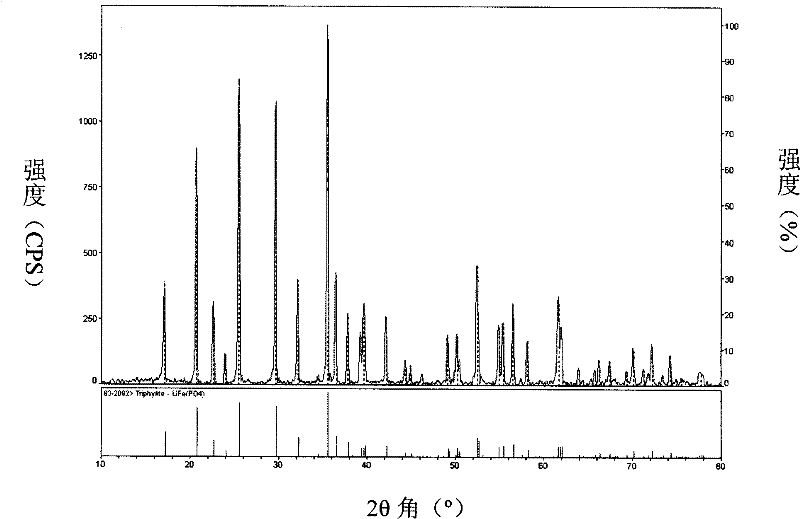
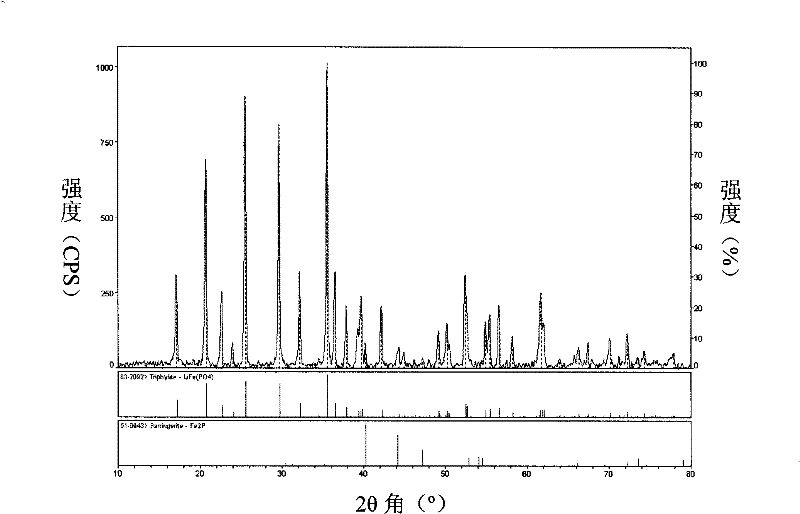
Method for preparing active substance lithium iron phosphate for lithium ion secondary battery anode
A cathode active material, lithium iron phosphate technology, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of low specific capacity, high battery internal resistance, lithium iron phosphate doping, etc., to achieve low internal resistance, good cycle performance, high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment illustrates the preparation of the positive electrode active material lithium iron phosphate provided by the invention
[0032] (1) 369 grams of Li 2 CO 3 , 1799 g FeC 2 o 4 2H 2 O. 1150 g NH 4 h 2 PO 4 Mix with 300 grams of glucose and 3000 grams of alcohol (Li:Fe:P molar ratio is 1:1:1), ball mill for 10 hours at a speed of 300 rpm, take it out, and dry it at 80°C;
[0033] (2) the mixture of step (1) is placed in a reaction vessel provided with an air inlet and an air outlet (the air inlet and the air outlet are on the same vertical plane, and the air inlet is down, and the air outlet is on the top), Open the gas outlet and the gas inlet, feed argon gas at a flow rate of 5 liters / minute, replace the air in the reaction vessel, then close the gas inlet, connect the gas outlet to the conduit and put the conduit into a 25°C Hydraulic oil (Caltex, special-grade hydraulic oil 460) (the conduit port is 5 cm below the hydraulic oil level), then heat ...
Embodiment 2
[0036] This embodiment illustrates the preparation of the positive electrode active material lithium iron phosphate provided by the invention
[0037] (1) 239.5 grams of LiOH, 1158.6 grams of FeCO 3 , 1319.7 grams (NH 4 ) 2 HPO 4 Mix 320 grams of glucose with 2700 grams of alcohol (Li: Fe: P molar ratio is 1:1:1), ball mill for 10 hours at a speed of 300 rpm, take it out, and dry at 80°C;
[0038] (2) the mixture of step (1) is placed in a reaction vessel provided with an air inlet and an air outlet (the air inlet and the air outlet are on the same vertical plane, and the air inlet is down, and the air outlet is on the top), Open the gas outlet and the gas inlet, feed argon gas at a flow rate of 5 liters / minute, replace the air in the reaction vessel, then close the gas inlet, connect the gas outlet to the conduit and put the conduit into a 25°C In the hydraulic oil (the conduit port is 5 cm below the hydraulic oil level), then heat up to 700 °C at a constant temperature f...
Embodiment 3
[0041] This embodiment illustrates the preparation of the positive electrode active material lithium iron phosphate provided by the invention
[0042] (1) 369 grams of Li 2 CO 3 , 1799 g FeC 2 o 4 2H 2 O. 1150 g NH 4 h 2 PO 4 Mix with 310 grams of sucrose and 3000 grams of alcohol (Li:Fe:P molar ratio is 1:1:1), ball mill for 10 hours at a speed of 300 rpm, take it out, and dry at 80°C;
[0043] (2) the mixture of step (1) is placed in a reaction vessel provided with an air inlet and an air outlet (the air inlet and the air outlet are on the same vertical plane, and the air inlet is down, and the air outlet is on the top), Open the gas outlet and the gas inlet, feed argon gas at a flow rate of 5 liters / minute, replace the air in the reaction vessel, then close the gas inlet, connect the gas outlet to the conduit and put the conduit into a 25°C In the hydraulic oil (the conduit port is 5 cm below the hydraulic oil level), then heat up to 750 °C at a constant temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| internal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com