Medicament composition and package for fast reducing uric acid in blood and use of anserine for fast reducing uric acid in blood
A pharmaceutical composition, anserine technology, applied in the direction of drug combination, blood diseases, tripeptide components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
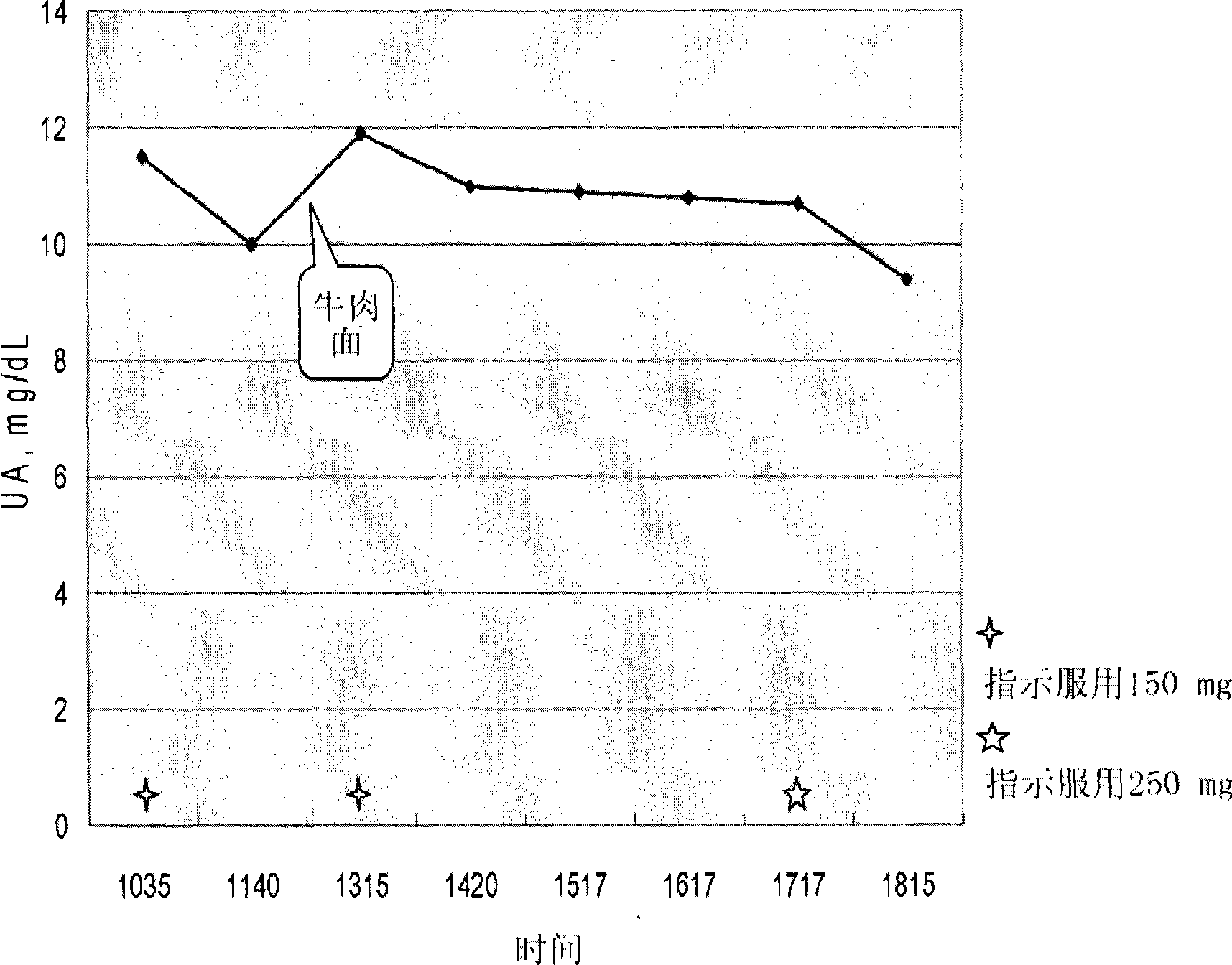
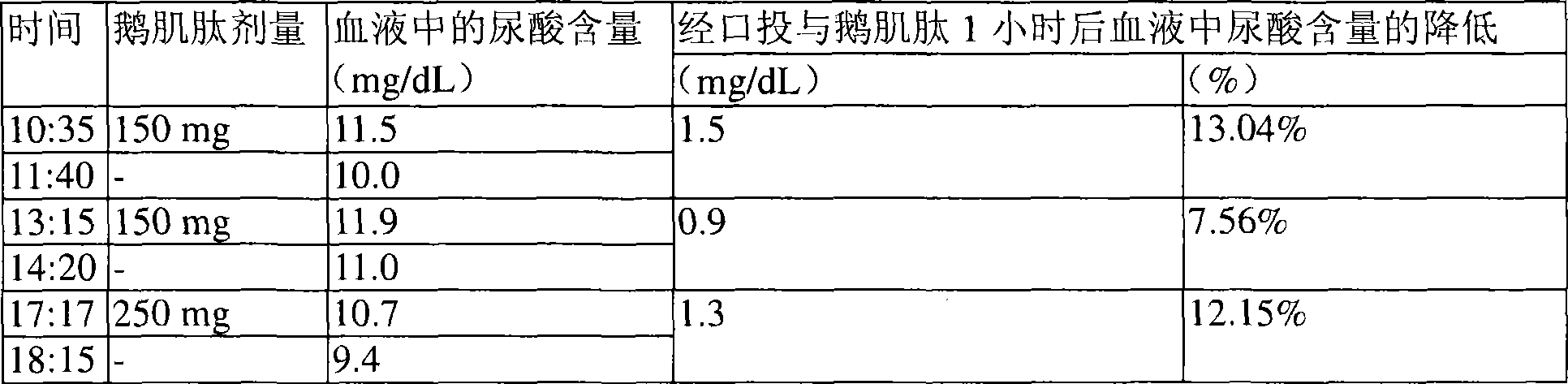
[0029]Anserine (GL Biochem Ltd., Shanghai, China) and dextrin-maltose were filled into gelatin capsules (total weight 400 mg), and the capsules contained 50 mg anserine. Individuals suffering from gout who have high levels of uric acid in the blood take 3 capsules (total anserine 150 mg) twice a day and 5 capsules once on the same day (total anserine 250 mg), so the individual takes daily The total dose of anserine is 550mg. The uric acid content in the blood was monitored by UroSpeed (a rapid serum-uric acid test strip, Apex Biotechnology Co., Inc., Hsinchu, Taiwan, ROC). The results are shown in Table 1 and further illustrated in figure 1 middle.
[0030] Table 1: Decrease in blood uric acid level 1 hour after oral administration of anserine
[0031]
[0032] as table 1 and figure 1 As shown, the reduction of uric acid levels in the blood ranged from 0.9 mg / dL to 1.5 mg / dL 1 hour after taking the capsules containing anserine. One hour after taking 150 mg of anserin...
example 2
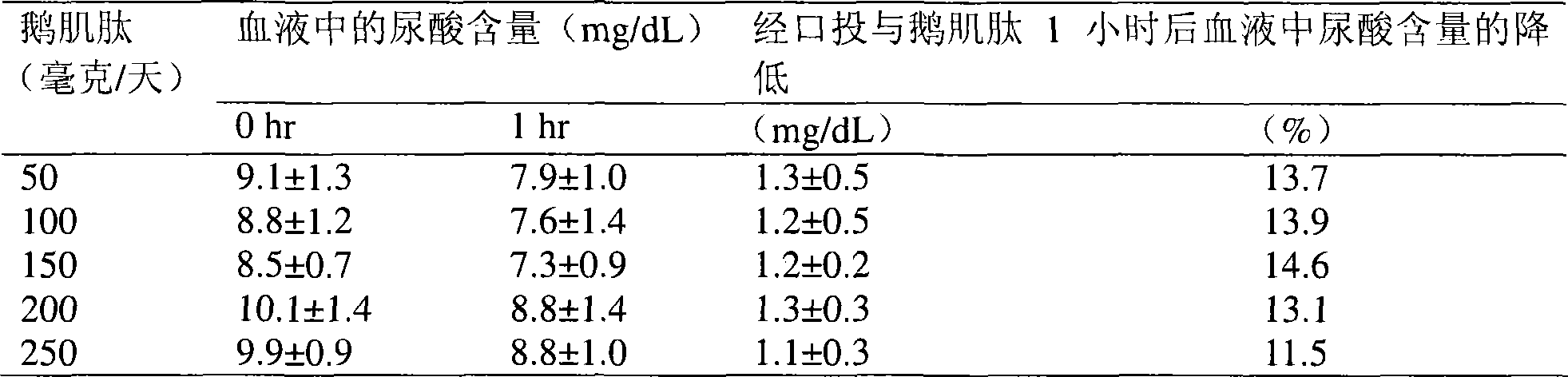
[0034] In this oral administration test, 15 voluntary adult subjects (9 males and 6 females aged 28 to 53) suffering from chronic hyperuricemia were tested. The individual discontinued their normal medication for at least 3 days. The average uric acid level in the blood of the individual is greater than 9.3 mg / dL. The individuals were randomly divided into 5 groups, each group comprising 3 individuals, who took the following doses of anserine respectively: 50 mg, 100 mg, 150 mg, 200 mg and 250 mg. After 1 hour, the level of uric acid in the individual's blood was measured. The uric acid level in his blood was found to decrease by 1.1 mg / dL to 1.3 mg / dL. Among the 5 groups, the group taking 150 mg of anserine had the most significant reduction in blood uric acid levels. The results are shown in Table 2.
[0035] Table 2: Effects of 1 hour after oral administration of various doses of anserine on the uric acid content in blood
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com