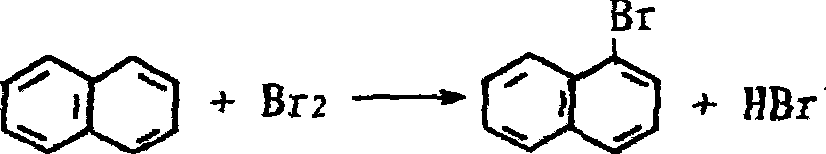Preparation of 1-naphthalene bromide
A technology of brominated naphthalene and refined naphthalene, which is applied in the field of preparation of 1-bromonaphthalene, can solve the problems of high raw material price and damage, and achieve the effect of reducing production cost and benefiting environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 1.03mol refined naphthalene and 125 grams of mixed dichloroethane solvent (1,2-dichloroethane and 1,1-dichloroethane's weight ratio is 30%:70%) in reactor, heat up under stirring Dissolve, add 1.13 mol of bromine dropwise, after the addition, reflux at 75-95°C for 5 hours, and the reaction ends. Then change to a distillation device, recover the mixed dichloroethane solvent, and completely distill the mixed dichloroethane. Then add 40 grams of 30% by weight sodium hydroxide to the residue in the bottle, and remove the alkali water layer to obtain 201 grams of crude product 1-bromonaphthalene, which is moved into a fractionating bottle for fractional distillation under reduced pressure. 158.5 g of the main fraction was obtained by distillation under reduced pressure at 135°C, with a yield of 74.02%, and the quality met the national chemical purity standard.
Embodiment 2
[0020] Add 1.03mol refined naphthalene and 110 grams of mixed dichloroethane solvent (1,2-dichloroethane and 1,1-dichloroethane's weight ratio is 50%:50%) in reactor, heat up under stirring Dissolve, add 1.08 mol of bromine dropwise, after the addition, reflux at 75-95°C for 5 hours, and the reaction ends. Then change to a distillation device, recover the mixed dichloroethane solvent, and completely distill the mixed dichloroethane. Add 30 grams of 30% by weight sodium hydroxide to the residue in the bottle, separate the aqueous alkali layer to obtain 202.5 grams of crude product 1-bromonaphthalene, which is moved into a fractionating bottle for fractional distillation under reduced pressure. 159.5 g of the main fraction was obtained by distillation under reduced pressure at 135°C, with a yield of 74.52%, and the quality met the national chemical purity standard.
Embodiment 3
[0022] Add 1.03mol refined naphthalene and 95 grams of mixed dichloroethane solvent (1,2-dichloroethane and 1,1-dichloroethane's weight ratio is 30%:70%) in reactor, heat up under stirring Dissolve, add 1.05 mol of bromine dropwise, reflux at 75-95°C for 5 hours after addition, and the reaction ends. Then change to a distillation device, recover the mixed dichloroethane solvent, and completely distill the mixed dichloroethane. Add 20 grams of 30% by weight sodium hydroxide to the residue in the bottle, separate the aqueous alkali layer to obtain 209 grams of crude product 1-bromonaphthalene, which is moved into a fractionating bottle for fractional distillation under reduced pressure. 164 g of the main fraction was obtained by distillation under reduced pressure at 135°C, with a yield of 76.62%, and the quality met the national chemical purity standard.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com