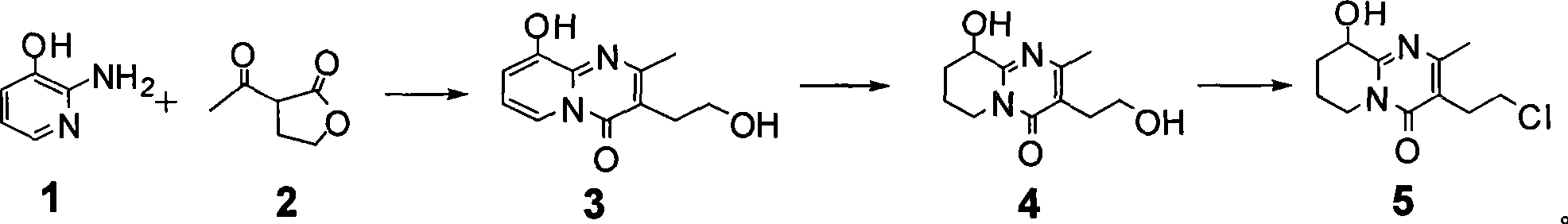
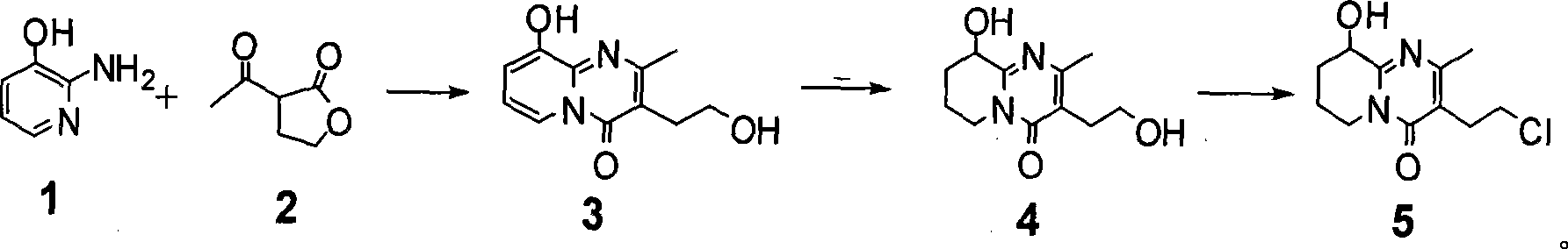
Process for synthesizing paliperidone intermediate
A technology of paliperidone and its synthetic method, which is applied in the field of preparation of raw material pharmaceutical chemical products, can solve the problems of incomplete cyclization, difficult industrialization, poor dehalogenation selectivity, etc., and achieves convenient separation, high selectivity, and easy separation and purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 1L four-necked reaction flask equipped with a stirring device, a thermometer and a reflux water separation device, sequentially add 66g of compound 1, 650mL of xylene, 84.5g of compound 2 and 3.4g of methanesulfonic acid, heat to reflux for 8 hours, and stop After heating, the temperature of the system was reduced to 110-120°C, and filtered while it was still hot, and the filtrate was allowed to stand overnight at room temperature, and then filtered to obtain a solid, which was vacuum-dried (10mmHg) at 40°C for 10 hours to obtain 99g of compound 3. 1 H-NMR (500MHz, CDCl 3 ): δ 2.04(s, 3H), 2.42(t, 2H, J=6.1Hz), 3.13(t, 2H, J=6.1Hz), 5.61(t, 1H, J=7.2Hz), 5.68(d, 1H , J=7.3Hz), 6.78 (d, 1H, J=7.0Hz), yield 75%;
[0028] In a 1L four-necked reaction flask equipped with a stirring device, a thermometer, a gas conduit and a reflux device, 75g of compound 3 and 750mL of methanol were sequentially added, and 10g of 5% rhodium carbon was added under nitrogen replacement ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com