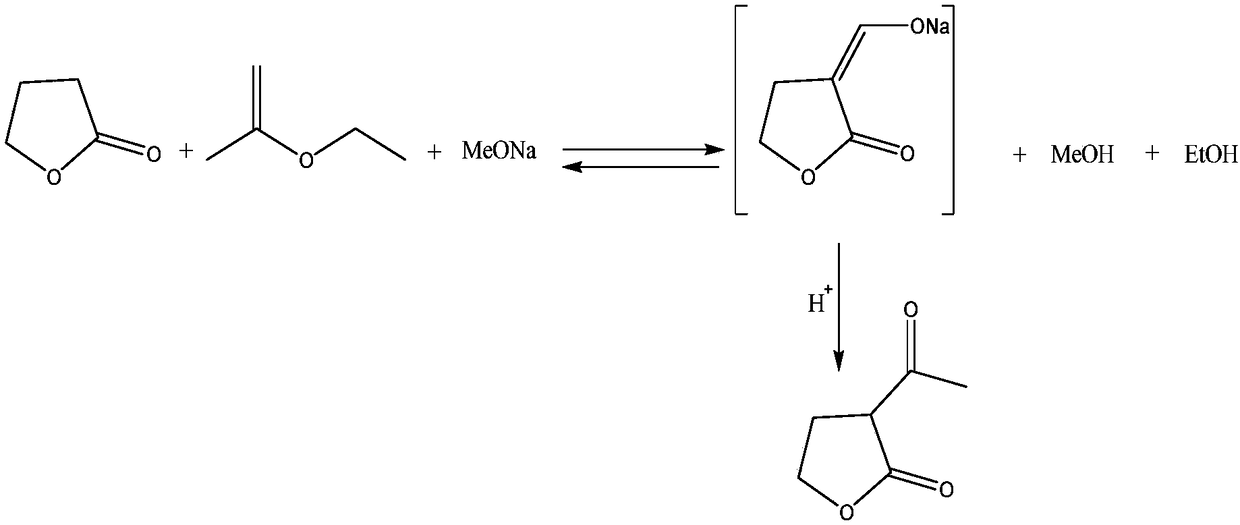
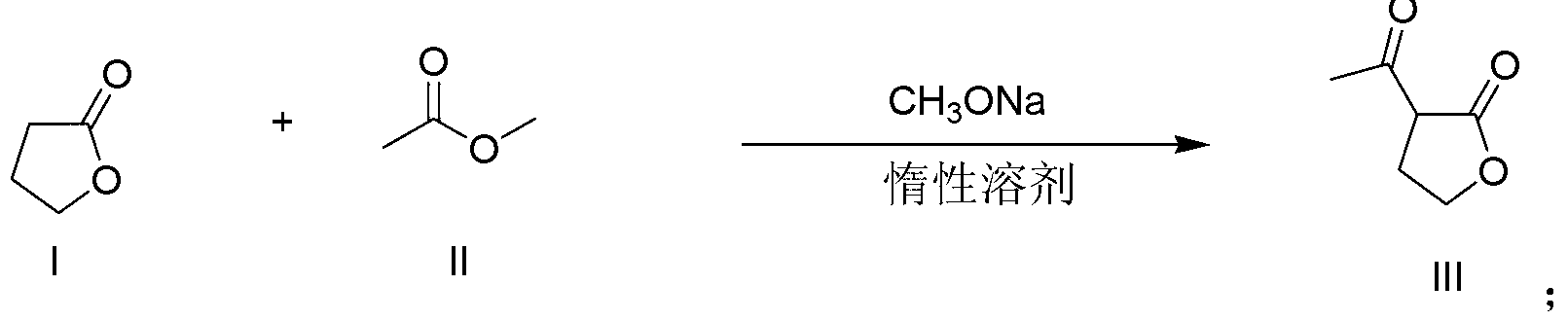
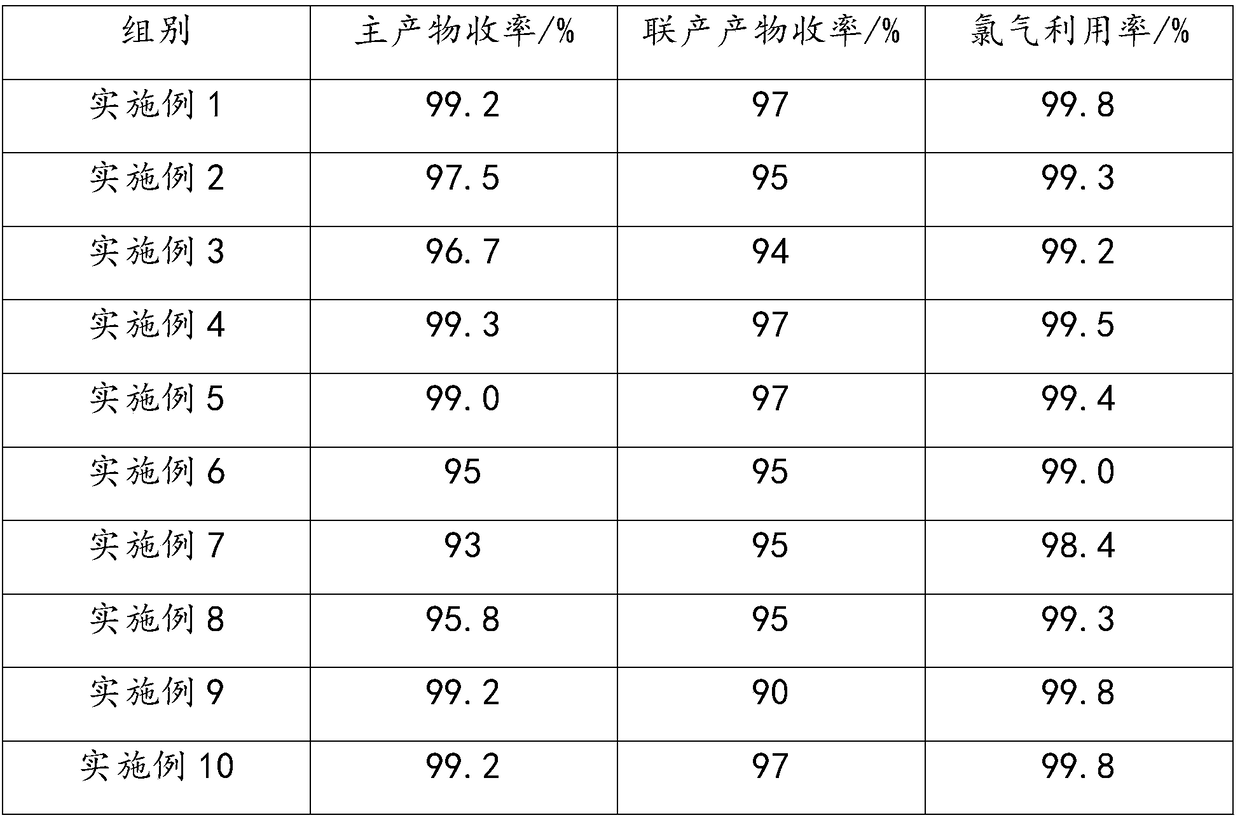
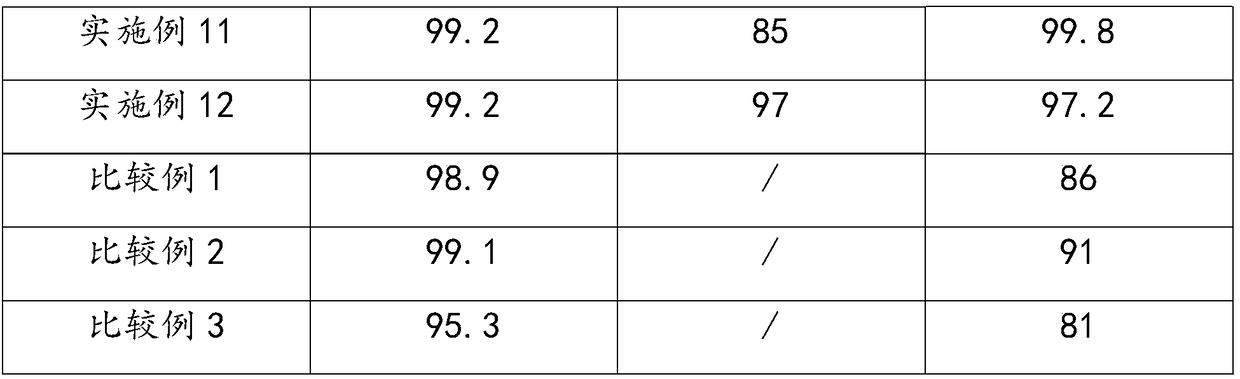
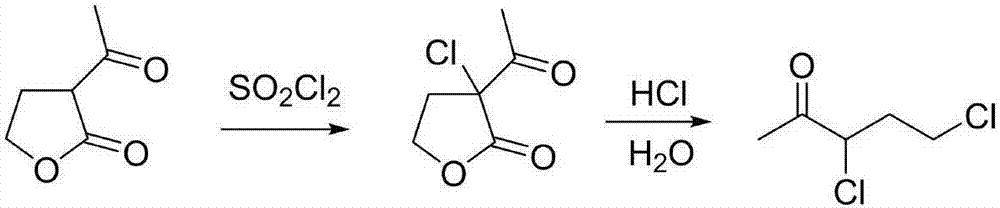
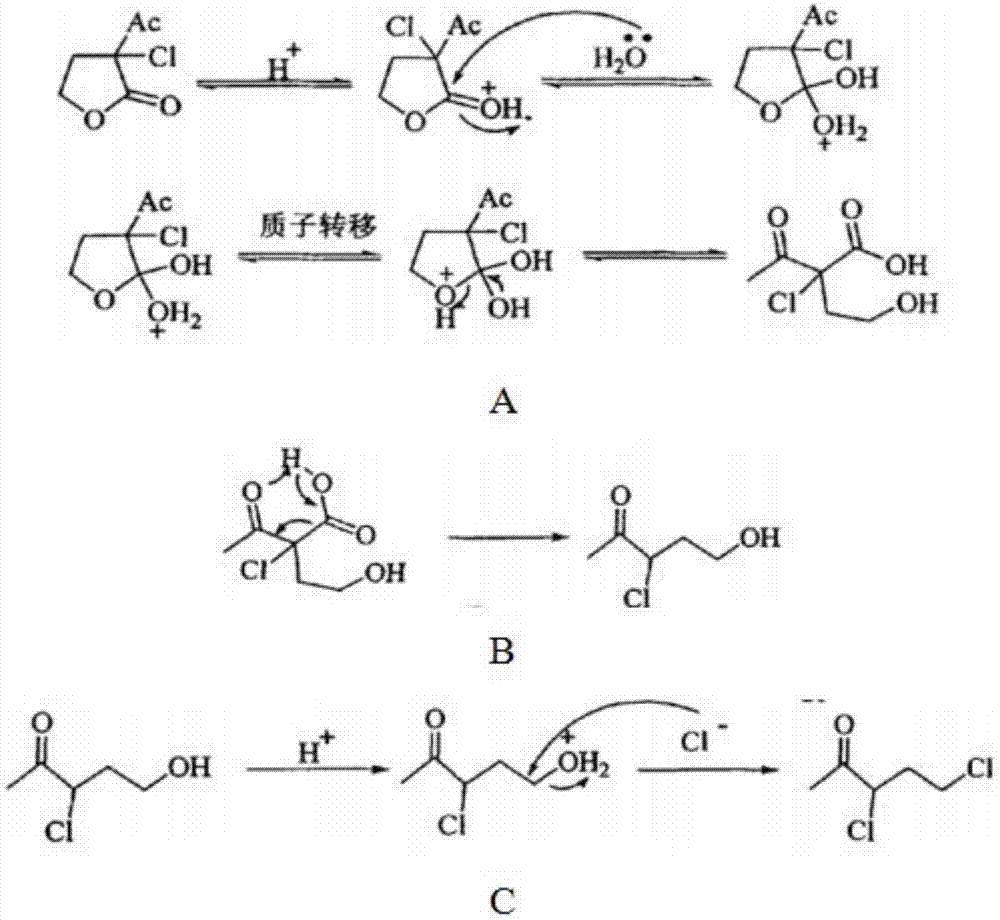
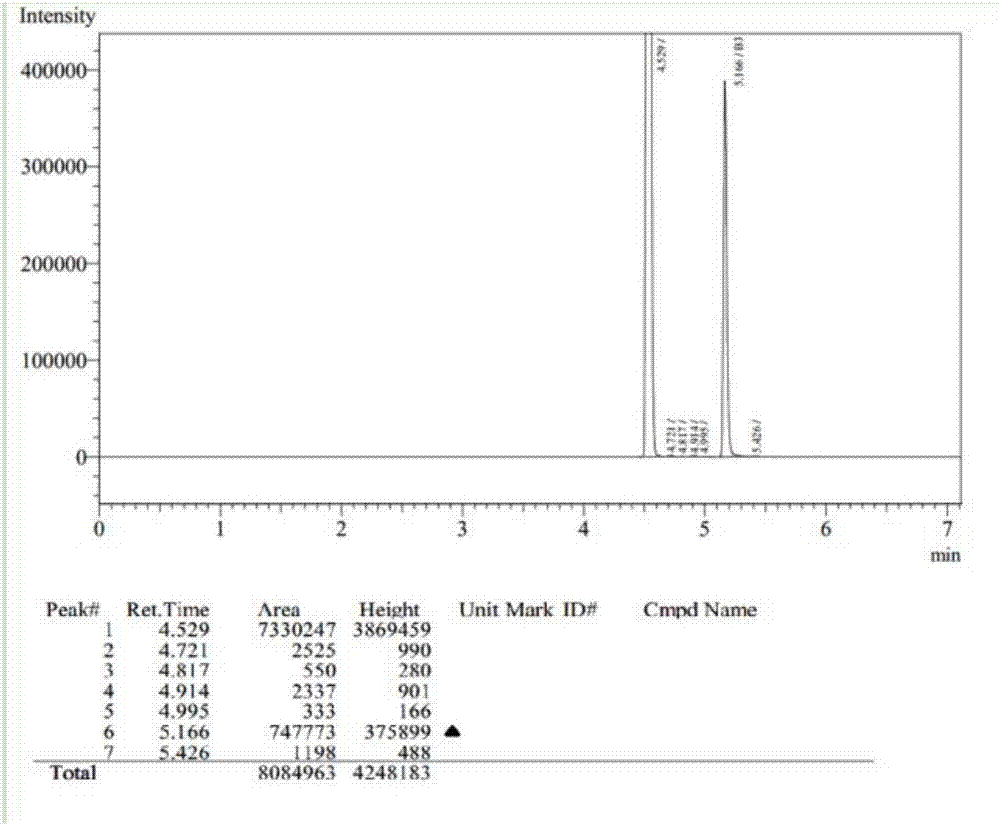
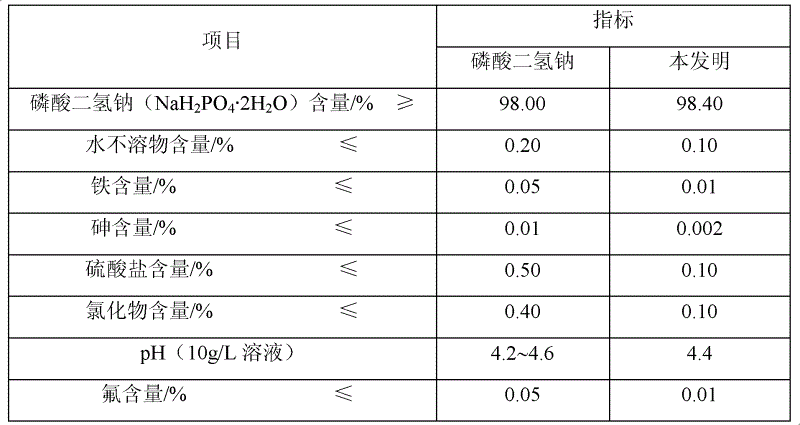
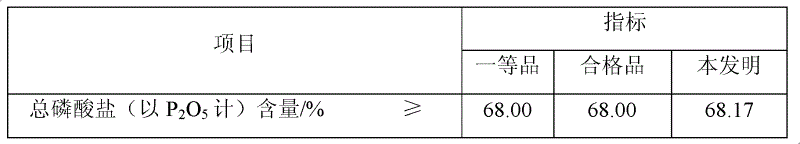
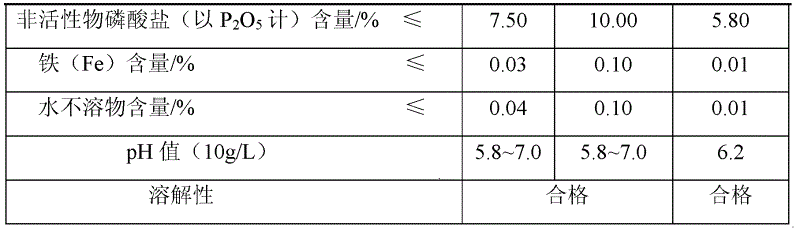
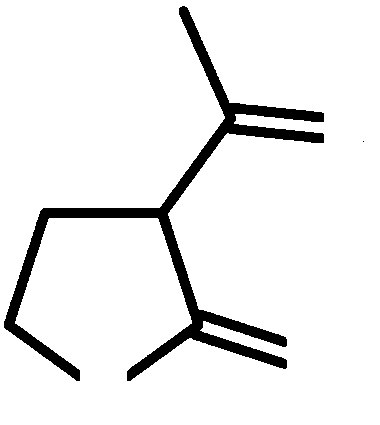
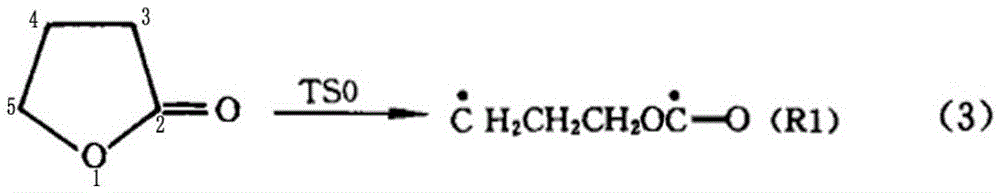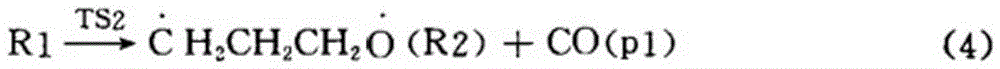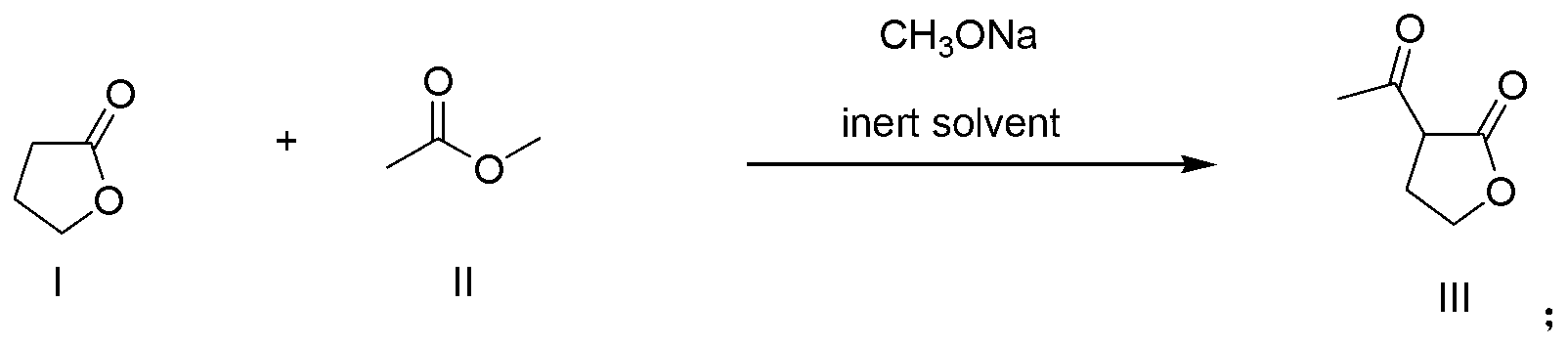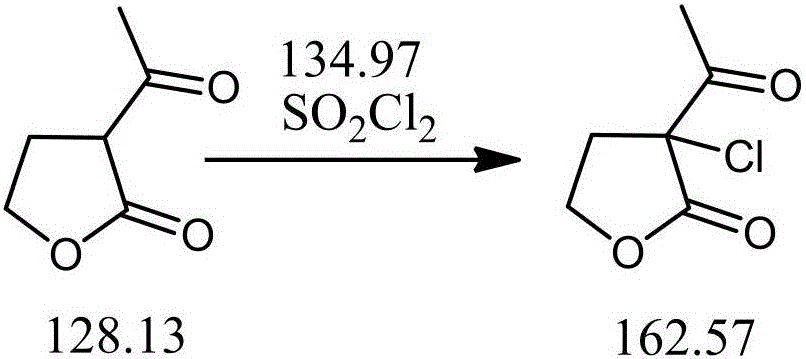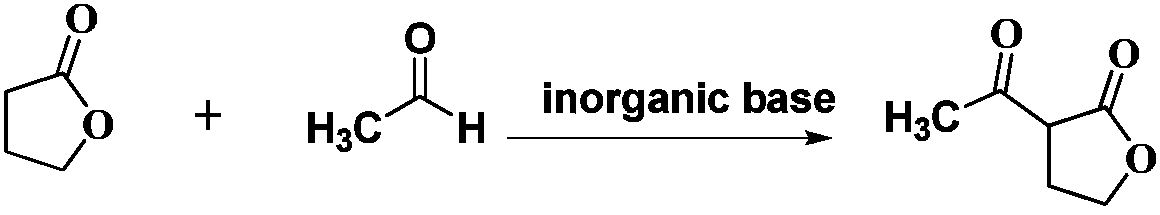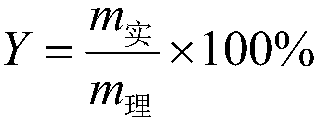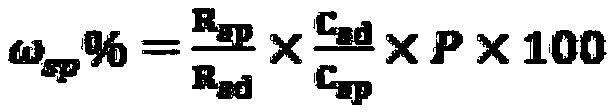Patents
Literature
44 results about "Alpha-acetyl-gamma-butyrolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
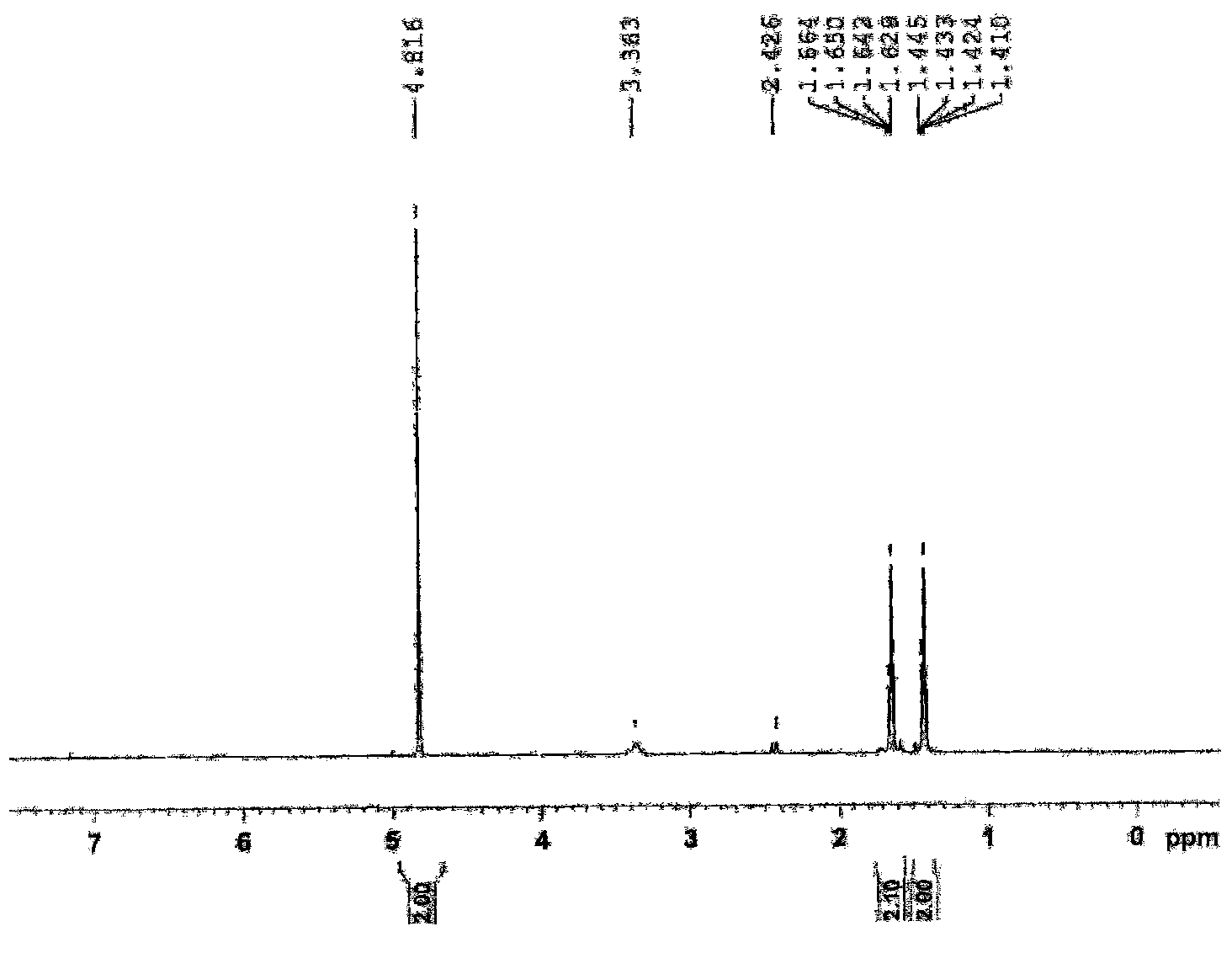
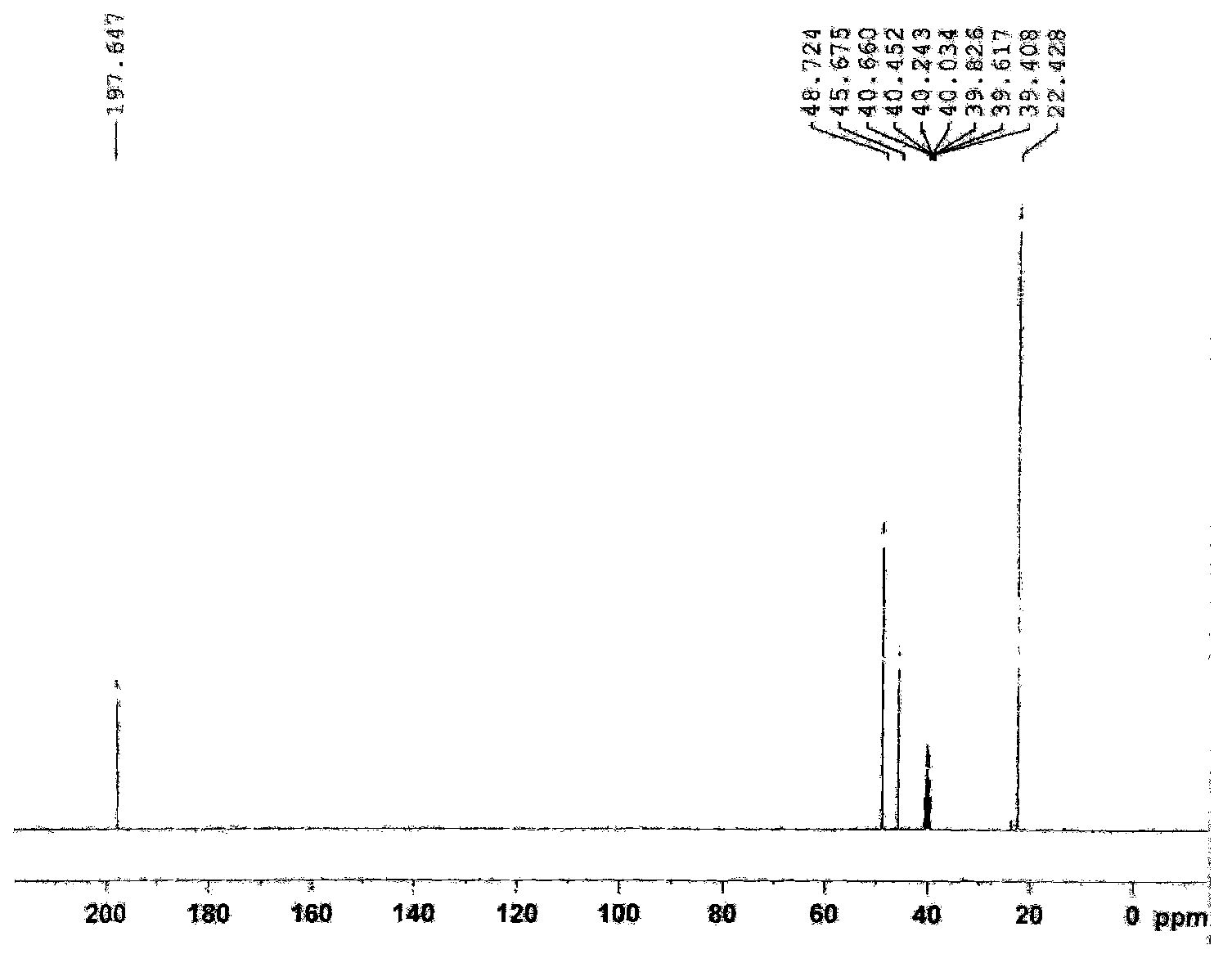
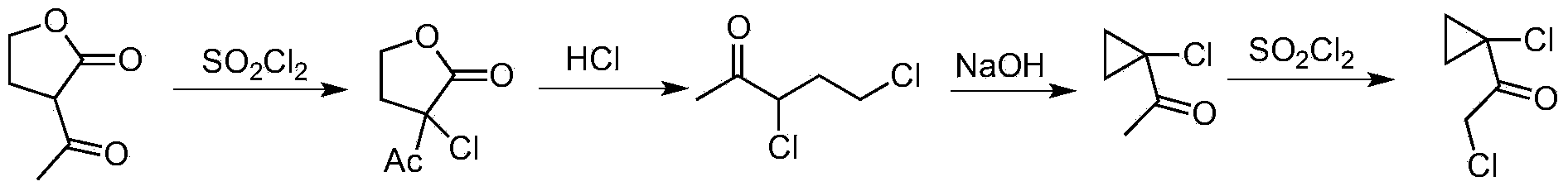
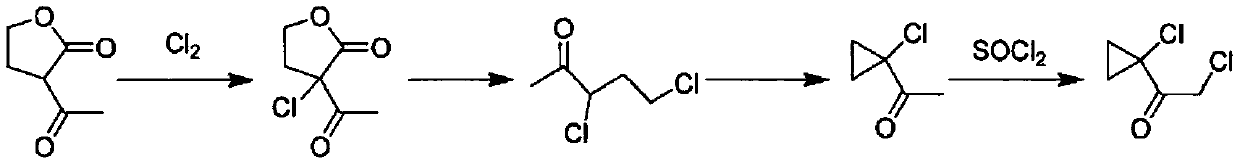
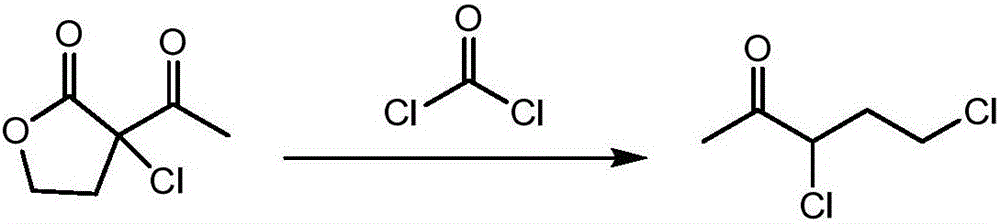
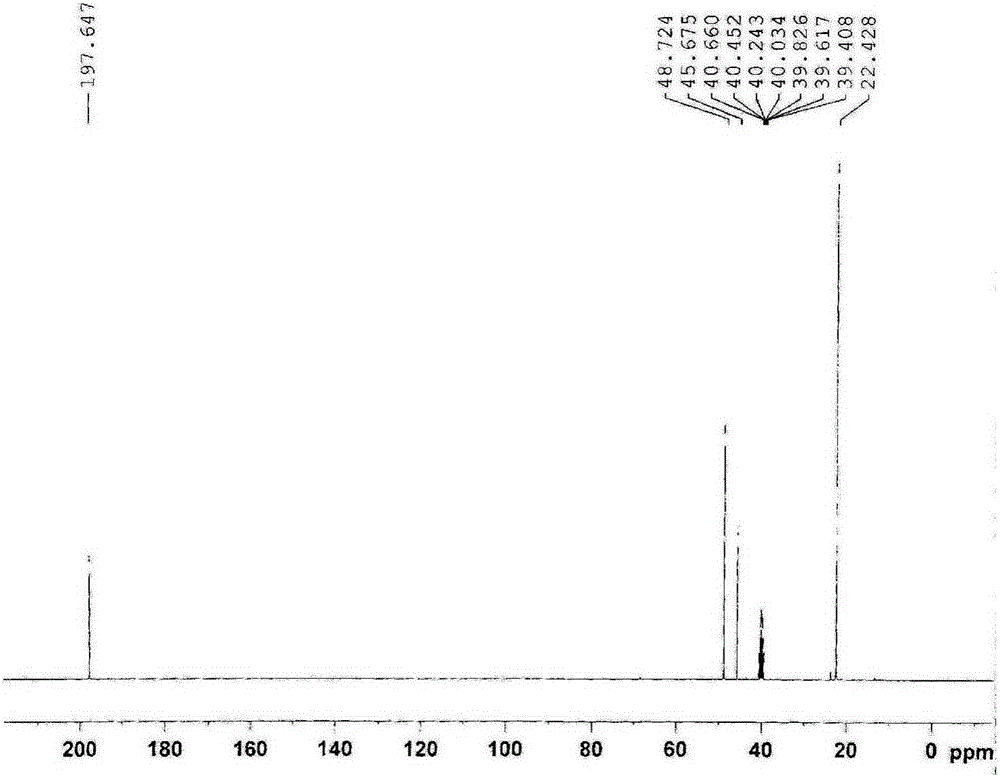
Synthetic process of 1-chloro-cyclopropanecarbonyl chloride
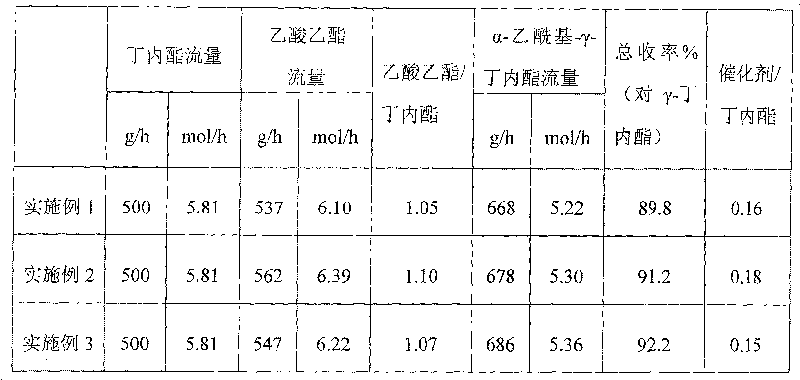
ActiveCN104292089ASmooth process connectionReduce unit operationsOrganic compound preparationPreparation from heterocyclic compoundsSulfonyl chlorideChloride
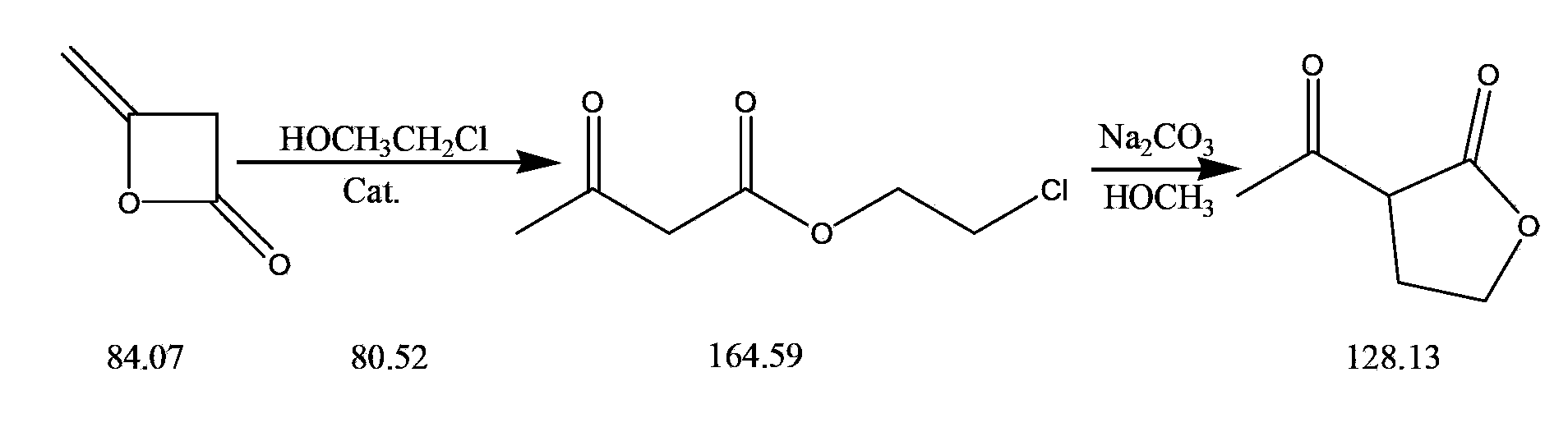
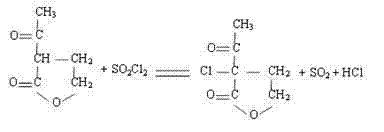
The invention discloses a synthetic process of 1-chloro-cyclopropanecarbonyl chloride, which belongs to the technical field of fine chemical processes. The process is implemented by taking alpha-acetyl-gamma-butyrolactone and sulfonyl chloride as raw materials through the steps of chlorinating, ring cleavage, cyclization, and re-chlorinating, so that a target product is obtained. The synthetic process is implemented by taking cheap industrial chemicals as raw materials and reaction reagents, and by using a single solvent system, a smooth process connection is achieved, so that the reaction yield is improved, the process operation is simplified, the cost of raw materials is reduced, and the cost of production is lowered.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
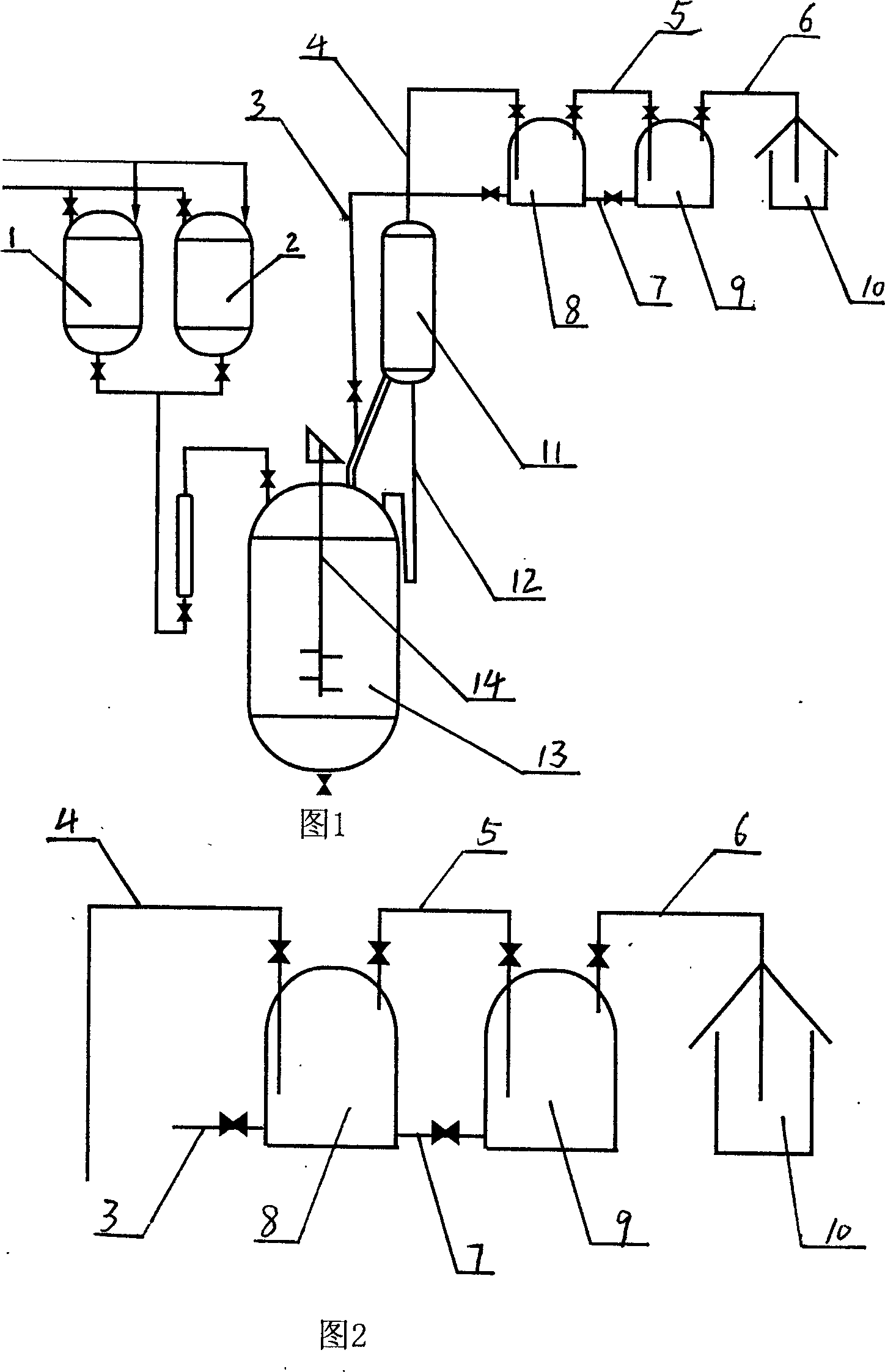
Prepn process and apparatus for alpha-acetyl-gamma-butyrolactone
InactiveCN1548427AAvoid safety hazardsEven contactOrganic chemistryAlpha-acetyl-gamma-butyrolactoneGamma-Butyrolactone
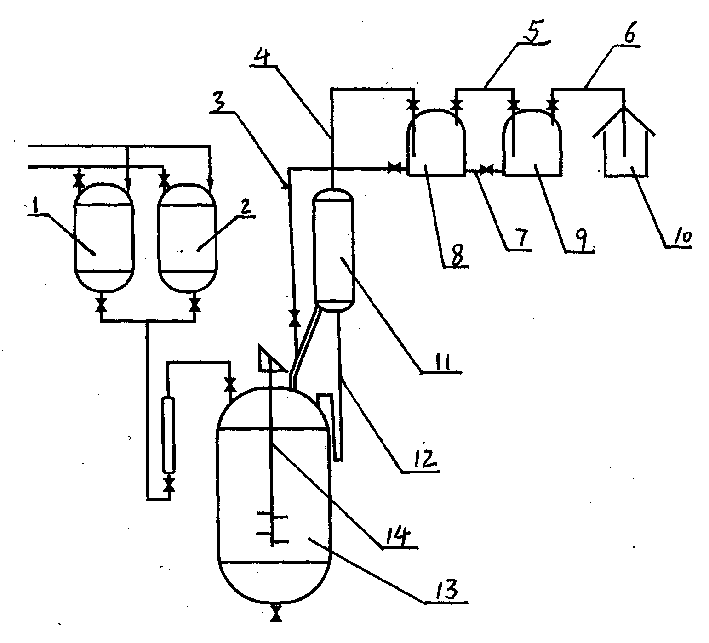
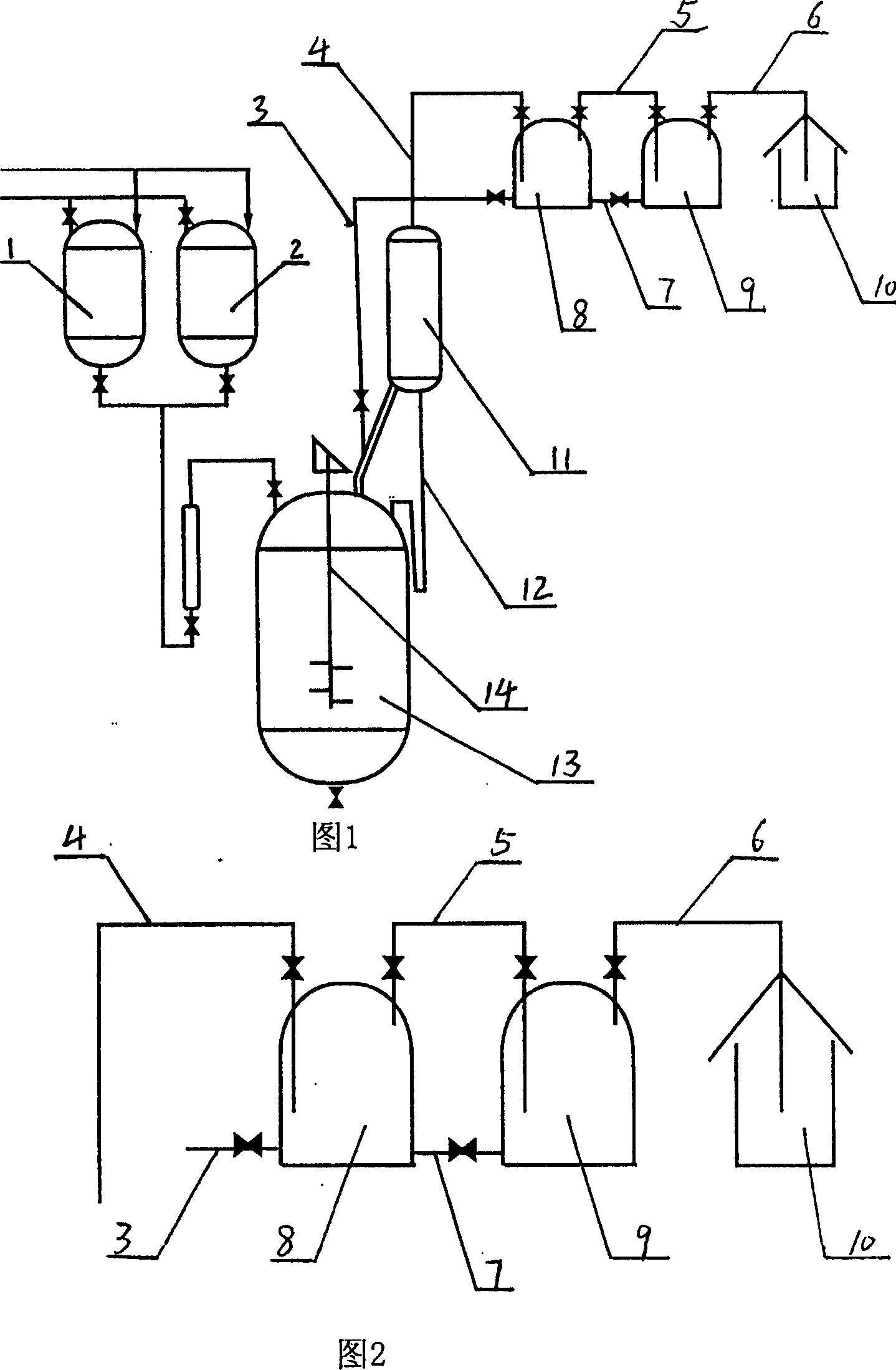
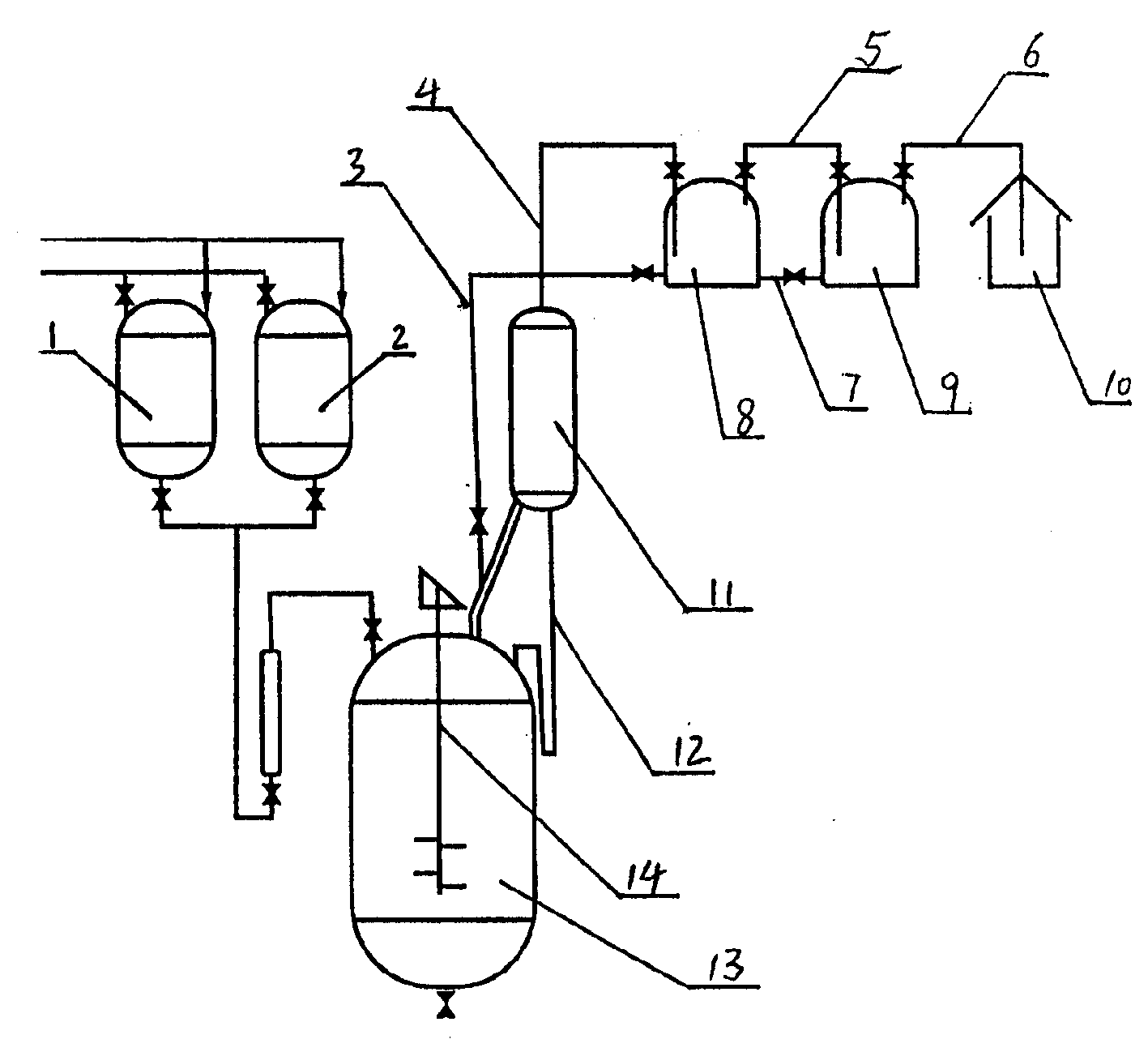
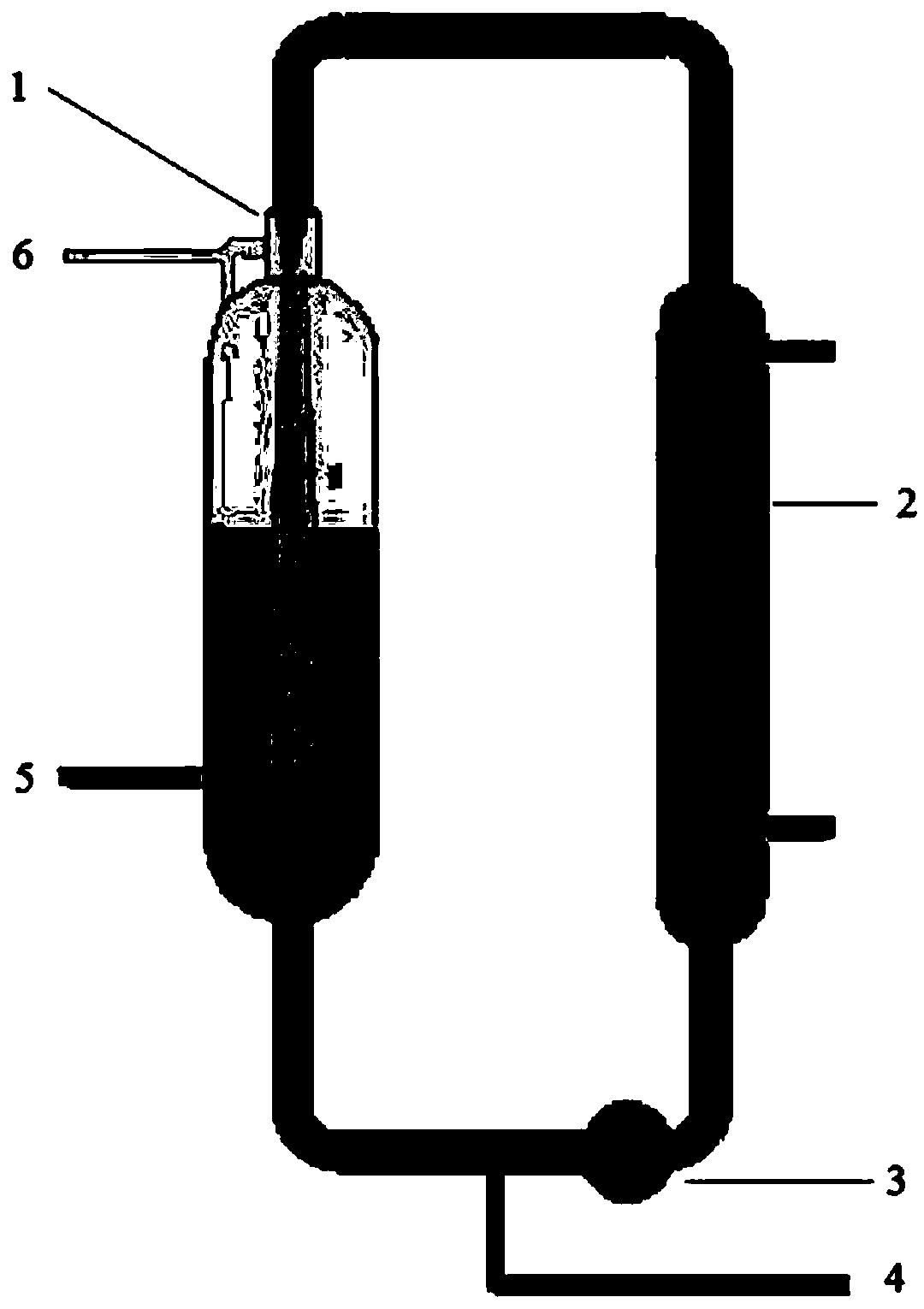
The preparation process of alpha-acetyl-gamma-butyrolactone includes reaction section, neutralization section and distillation section, and features the reaction section, which includes adding catalyst into benzene solvent, heating while stirring, fast cooling and dropping the liquid mixture of gamma-butyrolactone and acylating agent; the neutralization section before extraction; and the distillation section to prepare alpha-acetyl-gamma-butyrolactone. The present invention also relates to the reactor with one increased buffering reflux unit for preparing alpha-acetyl-gamma-butyrolactone.
Owner:LINHAI LIANSHENG CHEM
Method for preparing alpha-acetyl-gamma-butyrolactone
ActiveCN101768141AReduce pollutionQuick responseOrganic chemistryAlpha-acetyl-gamma-butyrolactoneEthyl fumarate
The invention provides a method for preparing alpha-acetyl-gamma-butyrolactone. In the method, gamma-butyrolactone and an acetylating agent (ethyl acetate and methyl acetate or a mixture of ethyl acetate and methyl acetate) are used as starting materials, metal sodium is used as a catalyst, and the product is obtained by acylation, neutralization and distillation. The method has the advantages that: the raw materials are used as the solvent at an initial stage of the reaction, so that the environmental pollution brought by the solvent is lowered, the material feeding amount is increased, the production efficiency is improved, and the production cost is lowered; the metal sodium is added into the reaction system continuously or for several times, so that potential safety risks such as outshoot and combustion caused by difficult control of the reaction speed can be effectively avoided when the sodium metal is reacted at a melting temperature; and by adopting the pressure-regulating distillation method, the byproducts are effectively recovered, the environmental pollution is lowered, and the comprehensive economic benefits are improved.
Owner:鞠彩霞
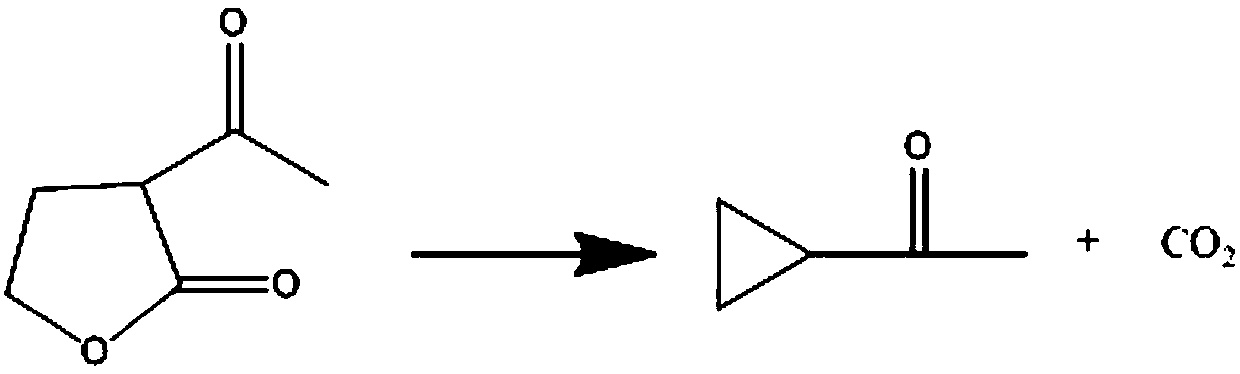
Method for preparing cyclopropyl methyl ketone
ActiveCN105622369AIncrease contact areaNot easy to wearCarbonyl compound separation/purificationPreparation from heterocyclic compoundsOrganic synthesisFixed bed
The invention relates to a method for preparing cyclopropyl methyl ketone, and belongs to the field of organic synthesis. The preparing method includes the following steps that metal halide and an inert solvent are added into a fixed bed reactor, the fixed bed reactor is heated to 185-195 DEG C, and then alpha-acetyl-gamma-butyrolactone is continuously added into the fixed bed reactor, and is subjected to a cleavage reaction; after the reaction is saturated, the alpha-acetyl-gamma-butyrolactone stops to be added, reaction distillation continues to be carried out till no product is distilled out, and a cyclopropyl-methyl-ketone crude product is obtained; the fixed bed reactor is connected with a rectifying tower, the prepared cyclopropyl-methyl-ketone crude product is transferred into the rectifying tower to be subjected to high tower dividing and then subjected to normal pressure rectification, the reflux ratio is adjusted, impurities are separated, and the high-purity cyclopropyl methyl ketone is obtained through distilling. The method is low in production cost, high in production efficiency, product yield and purity, small in by-product number, wastewater amount, waste material number and environment pollution and more suitable for industrial production.
Owner:LINHAI LIANSHENG CHEM
Preparation method for alpha-acetyl-gamma-butyrolactone
ActiveCN102229586AEfficient recyclingLow input costOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsReaction rateMethyl acetate
In order to solve the problems of a low safety factor, low yield, high cost, environmental pollution and the like existing in the prior art, the invention provides a preparation method for alpha-acetyl-gamma-butyrolactone. In the method, gamma-butyrolactone and methyl acetate are used as reaction raw materials and metallic sodium is used as a catalyst; the final product is prepared through the phases of reaction, separation, neutralization and extraction. According to the invention, methyl acetate is used as the reaction raw material and a reaction solvent, which on one hand enables production cost to be reduced, the reaction rate to be slowed and dashing out of reacting materials to be avoided, and on the other hand avoids environmental pollution and affection on product quality caused by utilization of benzene solvents; preparation of the catalyst into sodium sand can improve reaction efficiency; high purity alpha-acetyl-gamma-butyrolactone can be obtained through neutralization andextraction of the solid resultants alpha-acetyl-gamma-butyrolactone sodium salt of the reaction which have been subjected to solid-liquid separation without underpressure distillation, thereby effectively improving production efficiency and reducing production cost; the acidic neutral solution used in the invention can be used repeatedly, thereby avoiding environmental pollution by the solution.
Owner:山西三维华邦集团有限公司 +1
Method for preparing alpha ¿C acetyl ¿C gamma - butyrolactone
InactiveCN101092407AAvoid safety hazardsExtended service lifePhysical/chemical process catalystsOrganic chemistryAcetic acidTemperature control
This invention discloses a method for preparing alpha-acetyl-gamma-butyrolactone, which comprises a reaction process and a distillation process. The reaction process comprises: pumping gasified gamma-butyrolactone and ethyl acetate into a reaction loaded with catalyst, and reacting. The method utilizes gamma-butyrolactone and ethyl acetate to react, thus can avoid potential safety hazard caused by using ethylene oxide. The method utilizes supported solid catalyst and fixed bed reactor, and continuous gasification reaction mode to change previous intermittent reaction to continuous reaction, which can realzie uniform distribution of the reactants, prolonged service life of the catalyst, and shortened reaction time, and eliminate the deficiencies of slow heat dissipation and difficult temperature control of reaction kettle. The method has such advantages as stable and safe production, and high product yield and purity. The content of alpha-acetyl-gamma-butyrolactone is greater than or equal to 99.4%, the content of water is less than or equal to 0.15%, and the yield is greater than or equal to 92%.
Owner:泰州延龄精细化工有限公司
Method for preparing alpha-acetyl-gamma-butyrolactone
InactiveCN101230054AHigh metal activityHigh catalytic efficiencyOrganic chemistryNitrogen atmosphereEthyl ester
The invention relates to a preparation method for Alpha-acetyl-Gamma-butyrolactone. Ethyl acetate and Gamma-butyrolactone are used as the initial material, and the preparation method comprises a reaction section, a neutralization section and a distillation section. The reaction section comprises the following steps: firstly, a metallic sodium catalyst is added into a toluene solvent in nitrogen atmosphere, then the temperature is raised to 100 to 110 DEG C, and is maintained for 15 to 30 minutes; secondly, the mixed liquor of the ethyl acetate and the Gamma-butyrolactone is titrated into the toluene solvent after being treated in step one, heat preservation and reaction are performed for 2.5 to 3 hours at 85 to 90 DEG C, wherein, the mass compounding ratio between the ethyl acetate and the Gamma-butyrolactone is 1.55 to 1.70: 1; in the neutralization section, sulfuric acid is adopted for neutralization. Through the preparation method, high-purity Alpha-acetyl-Gamma-butyrolactone can be obtained, in addition, the operation of the method is simple, the production cost is low, and the security is good.
Owner:WUJIANG XINYI CHEM
Method for preparing alpha-acetyl-gamma-butyrolactone
The invention discloses a method for preparing alpha-acetyl-gamma-butyrolactone and relates to the technical field of chemical product preparation. The method comprises the following steps: by takingsolid sodium methoxide as a catalyst and gamma-butyrolactone and ethyl acetate as initial raw materials, implementing an acetylation reaction, after the reaction is completed, concentrating a reactionliquid, and separating an alpha-acetyl-gamma-butyrolactone sodium solid; implementing pulping and washing on the alpha-acetyl-gamma-butyrolactone sodium solid by using a second organic solvent in which the alpha-acetyl-gamma-butyrolactone sodium solid is not dissolvable; putting the washed alpha-acetyl-gamma-butyrolactone sodium solid into a third organic solvent, adjusting the pH value to 6-7 byusing an acid solution, stirring, filtering, and implementing vacuum distillation on filtrate, thereby obtaining alpha-acetyl-gamma-butyrolactone. The method has the characteristics of being short inpurification time, low in energy consumption, simple in preparation equipment, concise in operation, economically feasible, high in product purity, relatively high in yield, good in environment protection, and the like.
Owner:NORTHEAST PHARMA GRP +1
Preparation method of alpha-acetyl-gamma-butyrolactone
ActiveCN102030729AEasy to controlEase of industrial productionOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsVacuum pumpingCombustion
The invention provides a preparation method of an alpha-acetyl-gamma-butyrolactone. The method comprises the following steps: firstly, adding gamma-butyrolactone, an acetylating agent and a catalyst which are used as raw materials into an acylating kettle which is fully dried under the condition of stirring; and then, slowly heating, acylating, neutralizing and rectifying to obtain a product. The invention has the advantages that before reaction, all materials are added into the acylating kettle which is fully dried in advance, then the materials are slowly heated and acylated, and the acylating kettle has no need of vacuum pumping and nitrogen purging, thus the invention is simple and convenient in operation and is easy to realize industrialized production; by controlling the specific surface area of sodium metal and carrying out the acylation reaction under the mild condition that the temperature is lower than 90DEG C, serious potential safety hazards such as blanking, combustion, explosion and the like are completely eradicated, the occurrence of the side reaction is inhibited, the product has high purity and yield, and the by-product is simple in composition and can be recovered easily; and organic solvents are not used in the whole production process, thereby fundamentally eliminating the pollution source, and greatly lowering the production cost.
Owner:寿阳县世纪精细化工有限公司
Method for preparing alpha-acetyl-gamma-butyrolactone by using recycled reaction material
The invention relates to a method for preparing alpha-acetyl-gamma-butyrolactone by using a recycled reaction material, and particularly relates to a method for preparing high-yield alpha-acetyl-gamma-butyrolactone by reaction between recyclable cheap acylating agent and a catalyst in an inert solvent. The method comprises the six steps of acylation reaction, separation of reaction materials, neutral reaction, desolvation and distillation treatment, recovery treatment and cyclic utilization of a waste solvent. Full recycling of the materials is successfully achieved; the problem that a lot of methylbenzene-ethyl ester-ethanol ternary azeotropic solvent is generated in the traditional main technology at home is avoided; all materials can be recycled except for small loss in the reaction process; the environmental pollution is greatly reduced; and the preparation method accords with the national industrial policy at present, and is suitable for industrial popularization.
Owner:HAIZHENG CHEM NANTONG CO LTD
Continuous synthesis process of alpha-chloro-alpha-acetyl-gamma-butyrolactone
The invention relates to the technical field of chemical product preparation, and especially relates to a continuous synthesis process of alpha-chloro-alpha-acetyl-gamma-butyrolactone. The method comprises the steps that: alpha-acetyl-gamma-butyrolactone, water, and sodium bicarbonate are added into a same mixer; stirring is started, such that the materials are sufficiently mixed, and a premix liquid is prepared; the premix liquid is added into a constant-pressure premix liquid dropping funnel; through dropping flow meter metering, the liquid enters the bottom of a reaction kettle; chlorine gas is metered, and enters the bottom of the reaction kettle; a reaction temperature is controlled, such that a chlorination reaction is carried out; overflow liquid in the reaction kettle enters a stratification device through a discharge pipe, and stratification is carried out; upper-layer water layer is recovered through a water layer pipe, and a lower-layer finished product is recovered through a finished product pipe. According to the alpha-chloro-alpha-acetyl-gamma-butyrolactone continuous synthesis process, continuous production is realized, chlorine gas utilization rate is improved, product yield is improved, reaction rate is improved, and production capacity is improved.
Owner:江苏兄弟维生素有限公司
Method for preparing alpha - acetyl - gamma - butyrolactone
InactiveCN101092407BAvoid safety hazardsExtended service lifeOrganic chemistryPhysical/chemical process catalystsTemperature controlAcetic acid
This invention discloses a method for preparing alpha-acetyl-gamma-butyrolactone, which comprises a reaction process and a distillation process. The reaction process comprises: pumping gasified gamma-butyrolactone and ethyl acetate into a reaction loaded with catalyst, and reacting. The method utilizes gamma-butyrolactone and ethyl acetate to react, thus can avoid potential safety hazard caused byusing ethylene oxide. The method utilizes supported solid catalyst and fixed bed reactor, and continuous gasification reaction mode to change previous intermittent reaction to continuous reaction, which can realzie uniform distribution of the reactants, prolonged service life of the catalyst, and shortened reaction time, and eliminate the deficiencies of slow heat dissipation and difficult temperature control of reaction kettle. The method has such advantages as stable and safe production, and high product yield and purity. The content of alpha-acetyl-gamma-butyrolactone is greater than or equal to 99.4%, the content of water is less than or equal to 0.15%, and the yield is greater than or equal to 92%.
Owner:泰州延龄精细化工有限公司
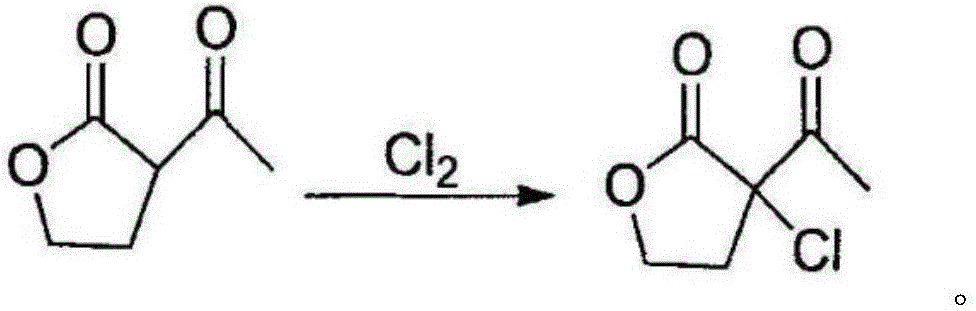
Preparation method of alpha-chloro-alpha-acetyl-gamma-butyrolactone
InactiveCN106588832AAvoid it happening againReduce harmOrganic chemistrySodium bicarbonateSulfonyl chloride
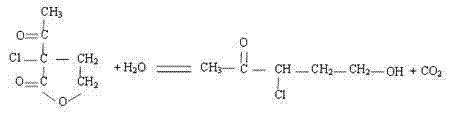
The invention discloses a preparation method of alpha-chloro-alpha-acetyl-gamma-butyrolactone. The method comprises the steps that alpha-acetyl-gamma-butyrolactone is cooled to low temperature, chlorine is introduced at the temperature, the saturation state of chlorine is kept, reacting is conducted for 5 h or above, after reacting is finished, introduction of chlorine is stopped, nitrogen is introduced to drive excess chlorine in a system and hydrogen chloride gas generated through reacting away, washing is conducted with a sodium bicarbonate aqueous solution, and a target product is obtained. Under the low temperature condition, alpha-acetyl-gamma-butyrolactone reacts with chlorine to generate the target product, a traditional chloride reagent such as sulfonyl chloride is substituted with cheap chlorine, generation of waste gas such as sulfur dioxide is avoided, the reaction time is shortened, aftertreatment of reacting is simplified, the yield of the product alpha-chloro-alpha-acetyl-gamma-butyrolactone is increased to 98% or above from 90%, the production cost is greatly lowered, and a positive effect is achieved for industrial development of prothioconazole.
Owner:LIANYUNGANG JINDUN AGROCHEMICAL CO LTD
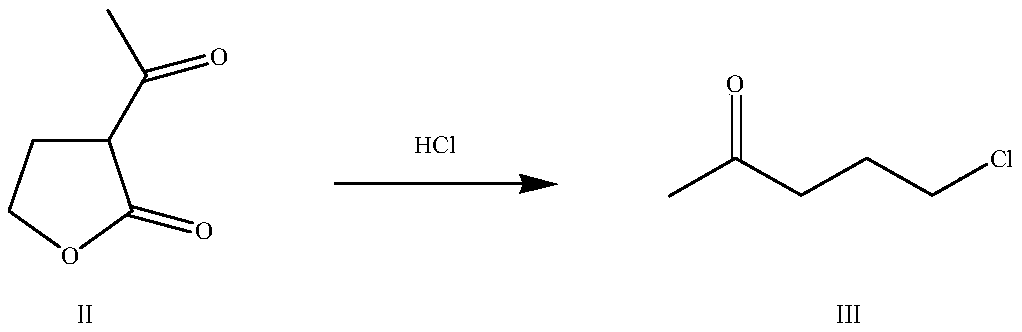
Preparation method of 2-chloro-1-(1-chlorocyclopropyl) acetone
InactiveCN109748783AShort reaction pathShort reaction cycleOrganic compound preparationCarbonyl compound preparationChemical synthesisChloride
The invention relates to the technical field of chemical synthesis, and particularly discloses a preparation method of 2-chloro-1-(1-chlorocyclopropyl) acetone. The preparation method comprises the following steps: enabling alpha-acetyl-gamma butyrolactone and hydrochloric acid to have an open-loop decarboxylic reaction to obtain 5-chloro-2-pentanone; adding the 5-chloro-2-pentanone and a catalystinto an alkaline solution, implementing a cyclization reaction, obtaining cyclopropyl methyl ketone; implementing a chlorination reaction with a chloride agent to obtain the 2-chloro-1-(1-chlorocyclopropyl) acetone. The reaction steps of the provided method are less, the reaction period is short, the yield is more than 70%, and the content of the 2-chloro-1-(1-chlorocyclopropyl) acetone in a product is more than 95%.
Owner:HEBEI CHENGXIN
Preparation method for Alpha-acetyl-Gamma-butyrolactone
ActiveCN108299345ALow costThe feeding situation is safe and environmentally friendlyOrganic chemistryOrganic solventAcetylation
The invention discloses a preparation method for Alpha-acetyl-Gamma-butyrolactone, and belongs to the technical field of compound synthesis. The method comprises the following steps: under the conditions of an organic solvent, using Gamma-butyrolactone and acetaldehyde as initial raw materials, using inorganic base as a catalyst, performing an acetylation reaction, adjusting a pH value of reactionliquid by using diluted acid until the reaction liquid is neutralized, and post-processing to obtain the Alpha-acetyl-Gamma-butyrolactone. The method has the characteristics of economical feasibility, higher yield, safety and environmental protection, and is suitable for industrialization.
Owner:沈阳东瑞精细化工有限公司
Method and device for synthesizing alpha-chloro-alpha-acetyl-gamma-butyrolactone for co-production of methyl formate
ActiveCN109400554ALow costAvoid pollutionPreparation from carboxylic acid saltsOrganic compound preparationChemical productsEthyl Chloride
The invention relates to the technical field of preparation of chemical products, in particular to a method and device for synthesizing alpha-chloro-alpha-acetyl-gamma-butyrolactone for co-productionof methyl formate. The method for synthesizing the alpha-chloro-alpha-acetyl-gamma-butyrolactone for the co-production of the methyl formate comprises the following steps of mixing alpha-acetyl-gamma-butyrolactone and a sodium formate aqueous solution, introducing chlorine gas for reaction and layering a reaction solution, wherein the lower layer substance is alpha-chloro-alpha-acetyl-gamma-butyrolactone, and making the upper layer substance to have esterification reaction with methanol to obtain the methyl formate. According to the method, the generation of gas like carbon dioxide is avoidedin the synthesis process, and the utilization rate of chlorine gas can be effectively improved. At the same time, a by-product can continuously react with methanol to produce the methyl formate afterthe certain reaction, and the by-product produced in the production process is fully utilized to meet the process demand, the production cost is greatly improved, and the environmental protection property is improved.
Owner:江苏兄弟维生素有限公司
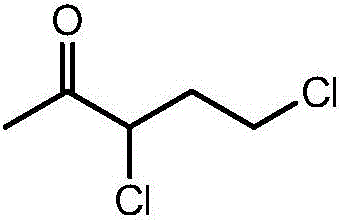
Synthesis process of 3,5-dichloro-2-pentanone
ActiveCN107473949AHigh purityAvoid it happening againPreparation from heterocyclic compoundsTemperature controlSulfonyl chloride
The invention discloses a synthesis process of 3,5-dichloro-2-pentanone. The process uses water as a solvent, alpha-acetyl-gamma-butyrolactone undergoes the continuous reaction of sulfonyl chloride chlorination, hydrolysis and ring opening, decarboxylation and re-chlorination in a double-layer glass reaction kettle to obtain the high-purity 3,5-dichloro-2-pentanone. The process adopts a staged temperature-controlled reaction, does not need any catalyst compared with a traditional process, can make the reaction completely performed, is safe and low in cost, is environmentally friendly, meanwhile is high in reaction yield and strong in industrialized operability and has a good application prospect.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Process for preparing alpha-acetyl-gamma-butyrolactone for co-production of various phosphates
InactiveCN102617520ARealize joint productionSimple conditionsOrganic chemistryPhosphorus compoundsEcological environmentFiltration
The invention provides a process for preparing alpha-acetyl-gamma-butyrolactone for co-production of various phosphates. The process is applicable to producing the alpha-acetyl-gamma-butyrolactone through acylation and phosphoric acid neutralization of gamma-butyrolactone and acetyl reagents serving as raw materials under the catalytic action of metallic sodium, and effective separation of an organic phase and an aqueous phase is achieved through controlling the concentration of phosphoric acid and the system temperature during oil-water separation. The organic phase is rectified to obtain alpha-acetyl-gamma-butyrolactone; and sodium dihydrogen phosphate dihydrate, sodium hexametaphosphate, sodium trimetaphosphate, sodium acid pyrophosphate, sodium dihydrogen phosphate dodecahydrate, trisodium phosphate dodecahydrate, sodium tripolyphosphate and sodium pyrophosphate are obtained through steps such as pH adjustment, concentration, decompressional filtration and the like of the aqueous phase. The process produces both main products and byproducts, achieves co-production of various products by one process, energy conservation, emission reduction and ecological environment protection, and is safe in operation, simple in process, easy to industrialize and low in cost.
Owner:SHANXI UNIV +1
Synthetic method for alpha-acetyl-gamma-butyrolactone
InactiveCN103387556AReduce usageReduce security risksOrganic chemistryChemical products2-Chloroethanol
The invention relates to the technical field of chemical product preparation, and particularly relates to a synthetic method for alpha-acetyl-gamma-butyrolactone. The synthetic method comprises the steps of carrying out an aldol reaction on ketene dimer and 2-chloroethanol under the effect of a catalyst sodium alkoxide to obtain acetyl chloride ethanol acetate; preparing alpha-acetyl-gamma-butyrolactone: adding a solvent to acetyl chloride ethanol acetate, mixing with stirring, carrying out an intramolecular Claisen-Schmidt condensation reaction under the effect of an alkali catalyst, and directly generating alpha-acetyl-gamma-butyrolactone by cyclization. The synthetic method for alpha-acetyl-gamma-butyrolactone can prevent use of dangerous chemical products such as ethylene oxide, metallic sodium and the like and greatly reduce security risks. Besides, the synthetic yield is obviously higher than that of a conventional process, and production cost is significantly reduced.
Owner:江苏兄弟维生素有限公司
Method for preparing alpha-acetyl-gamma-butyrolactone
InactiveCN108658902ARaw materials are easy to getSave raw materialsOrganic chemistryEvaporationHigh pressure
The invention discloses a method for preparing alpha-acetyl-gamma-butyrolactone. The method comprises the following steps: adding ethyl acetate and sodium carbonate into a three-necked flask, dropwiseadding gamma-butyrolactone at the temperature of 44 to 46 DEG C, stirring, mixing the ethyl acetate and liquid aldehyde, dropwise adding a reaction solution, uniformly mixing, stirring and reacting for 1.5 to 2.5 hours at the temperature of 54 to 58 DEG C, adding the reaction solution into a high-pressure kettle, reacting for 5 to 6 hours at the temperature of 80 to 85 DEG C, cooling to room temperature, adding the reaction solution into a three-necked flask, dropwise adding sulfuric acid at 3 to 5 DEG C to adjust the pH to be 6 to 7, precipitating solids in the dropwise addition process, after the addition is ended, stirring for 11 to 13 hours, re-testing the pH which is unchanged, filtering to remove the solids, washing a filter cake by using ethyl acetate, performing the decompressionrotary evaporation, removing the ethyl acetate until no fraction is outputted, obtaining a crude product, and performing the direct decompression rectification for the crude product. The preparation method is easy in obtaining raw materials, cheap in raw materials, easy in operation, higher in yield, and suitable for industrialized production.
Owner:陈正新
Method for synthesizing 4-methyl-5-(2- ethoxy) thiazole
InactiveCN103772313AThe process steps are simpleImprove product qualityOrganic chemistryThiazoleThiourea
The invention provides a method for synthesizing 4-methyl-5-(2- ethoxy) thiazole. According to the method, the 4-methyl-5-(2- ethoxy) thiazole is prepared by using alpha-acetyl-gamma-butyrolactone through oxidation, hydrolyzed decarboxylation, synthesis and other steps. Thioformamide is utilized to replace thiourea in the method, so that the diazotizing and hydrolyzing steps for removing amidogen which is generated on a thiazole ring due to use of thiourea can be omitted, the process steps are simple, the product quality can be improved, and the production cost can be reduced.
Owner:BENGBU COLLEGE
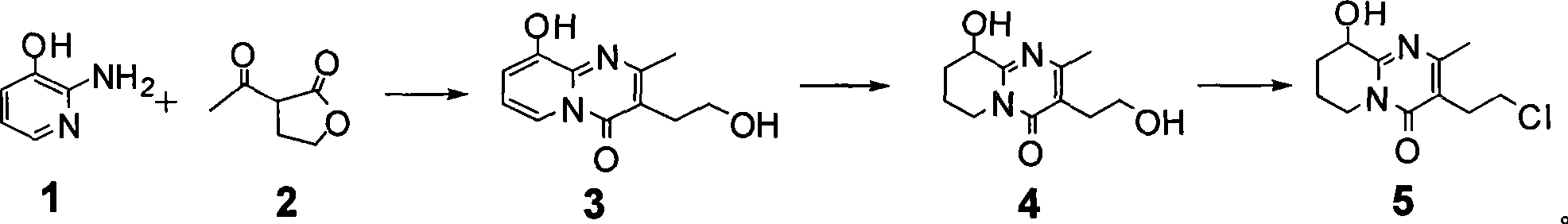
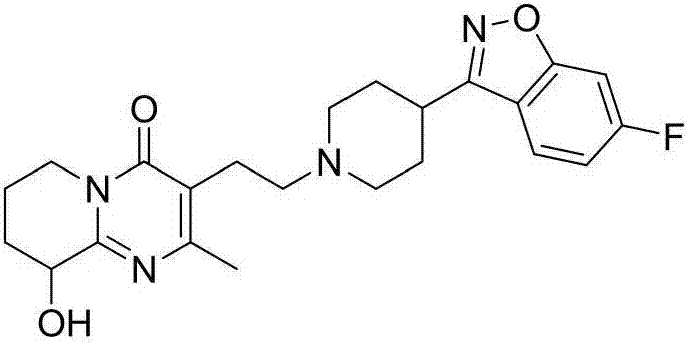
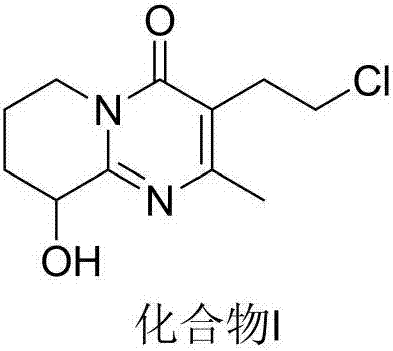
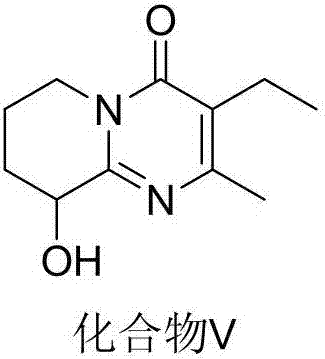
Process for synthesizing paliperidone intermediate
The invention relates to a method for synthesizing an important intermediate of paliperidone. The method relates to the intermediate of 3-(2-chloroethyl)-6, 7, 8, 9-tetrahydrochysene-9-oxhydryl-2-methyl-4H-pyridino-(1, 2-a)pyrimidine-4-kektone, and comprises: step one, in a solvent, catalytically cyclizing 2-amido-3-oxhydryl-pyridine and alpha-acetyl-gamma-butyrolactone by acid to obtain a compound 3; step two, in the solvent, catalytically hydrogenating a pyridine ring of the compound 3 by a transitional metal catalyst to obtain a compound 4; and step three, in the solvent, selectively chlorinating primary alcohol of the compound 4 by a chlorination reagent to obtain the intermediate of the paliperidone 3-(2-chloroethyl)-6, 7, 8, 9-tetrahydrochysene-9-oxhydryl-2-methyl-4H-pyridino-(1, 2-a)pyrimidine-4-kektone. The method has the characteristics of high selectivity and easy operation; a product and the intermediate can be easily separated and purified; and a compound 1 and a compound 2 can be cyclized completely.
Owner:SHANGHAI RECORD PHARM CO LTD
Continuous synthetic method for 3,5-dichloro-2-pentanone
ActiveCN106673978AReduce usageLower synthesis costPreparation from heterocyclic compoundsMicroreactorCarbonyl chloride
The invention discloses a continuous synthetic method for 3,5-dichloro-2-pentanone. The method comprises the steps of adding a dichloroethane solution of alpha-chloro-alpha-acetyl-gamma-butyrolactone and carbonyl chloride into a microreactor, and conducting continuous chlorination ring-opening reaction to generate 3,5-dichloro-2-pentanone. The continuous synthetic method for 3,5-dichloro-2-pentanone has the advantages of no catalyst, simplicity in operation, high reaction selectivity, low energy consumption, high purity, high yield and the like.
Owner:HUNAN CHEM RES INST
Preparation process and apparatus for alpha-acetyl-gamma-butyrolactone
InactiveCN1263749CAvoid safety hazardsEven contactOrganic chemistryAlpha-acetyl-gamma-butyrolactoneGamma-Butyrolactone
The preparation process of alpha-acetyl-gamma-butyrolactone includes reaction section, neutralization section and distillation section, and features the reaction section, which includes adding catalyst into benzene solvent, heating while stirring, fast cooling and dropping the liquid mixture of gamma-butyrolactone and acylating agent; the neutralization section before extraction; and the distillation section to prepare alpha-acetyl-gamma-butyrolactone. The present invention also relates to the reactor with one increased buffering reflux unit for preparing alpha-acetyl-gamma-butyrolactone.
Owner:LINHAI LIANSHENG CHEM
Recovery process and separation device of toluene solvent in production process of alpha-acetyl-gamma butyrolactone
InactiveCN102992930BContinuous operationReduce production energy consumptionOrganic compounds purification/separation/stabilisationOrganic compound preparationAcetic acidAlcohol
The invention relates to a recovery process and a separation device of a toluene solvent in the production process of alpha-acetyl-gamma butyrolactone. The recovery process comprises the following steps of: feeding from the upper part and lower part of a toluene extraction rectifying tower, so as to obtain a mixture of ethyl acetate, ethanol and a little toluene at the tower top and a mixture of toluene and ketone extracting agent, feeding the mixture in the tower kettle into a ketone extracting agent recovery tower for reduced pressure distillation so as to obtain toluene separated from the tower top and a toluene extraction agent separated from the tower kettle and returning the toluene extraction agent to a feed inlet on the upper part of a toluene extraction rectifying tower; feeding the material on the top of the toluene extraction rectifying tower into a toluene secondary extraction rectifying tower, recycling a ketone extraction agent and a little toluene in the tower kettle to the toluene extraction rectifying tower so as to obtain the qualified ethyl acetate at the tower top and a mixture of alcohol extraction agent and ethanol in the tower kettle, and feeding the mixture in the tower kettle into an alcohol extracting agent recovery tower for reduced pressure distillation so as to obtain ethanol separated from the tower top, and an alcohol extracting agent separated from the tower kettle. By adopting the extractive distillation technology and the separation device, the continuous production is achieved, and the toluene generated in the production process of alpha-acetyl-gamma butyrolactone is recovered.
Owner:FUZHOU UNIV
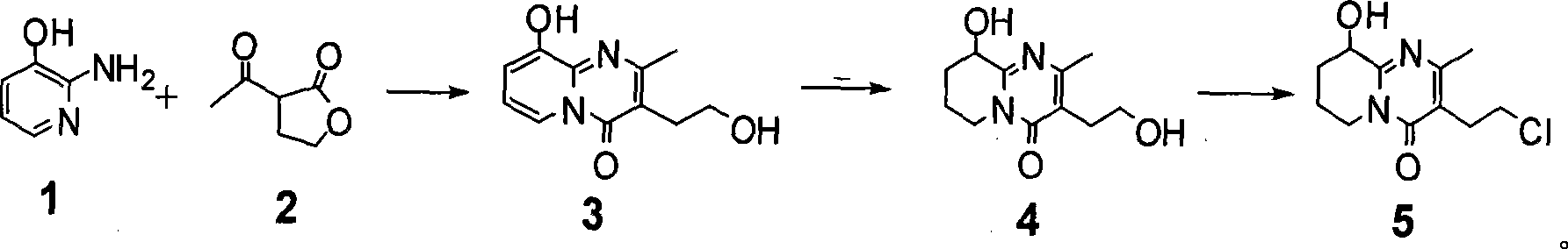
Preparation method of high-purity paliperidone intermediate
The invention belongs to the technical field of medicine, and particularly discloses a preparation method of a high-purity paliperidone intermediate 3-(2-chloroethyl)-9-hydroxyl-2-methyl-6,7,8,9-tetrahydro-4H-pyridino[1,2-a] pyrimidine-4-ketone. The preparation method particularly comprises the following steps: preparing 9-(benzyloxy)-3-(2-chloroethyl)-2-methyl-4H-pyridino[1,2-a] pyrimidine-4-ketone by taking 2-amino-3-hydroxypyridine as a starting raw material and through benzyl protection, ring closure of alpha-acetyl-gamma-butyrolactone and chloro-substitution; dissolving the 9-(benzyloxy)-3-(2-chloroethyl)-2-methyl-4H-pyridino[1,2-a] pyrimidine-4-ketone into an alcohol solvent, adding acid and a palladium-charcoal catalyst, controlling hydrogen pressure, and after the reaction is completed, performing processes of filtering, concentrating, adjusting the pH value, extracting, concentrating and refining to obtain white-like powdered 3-(2-chloroethyl)-9-hydroxyl-2-methyl-6,7,8,9-tetrahydro-4H-pyridino[1,2-a] pyrimidine-4-ketone. The preparation method is short in cycle, the product is white-like, the yield reaches 70 percent or higher, the purity reaches 99 percent or higher, related impurities of chlorine-removed byproducts are avoided, and the preparation method is suitable for industrialized enlarged production.
Owner:JINAN KANGHE MEDICAL TECH
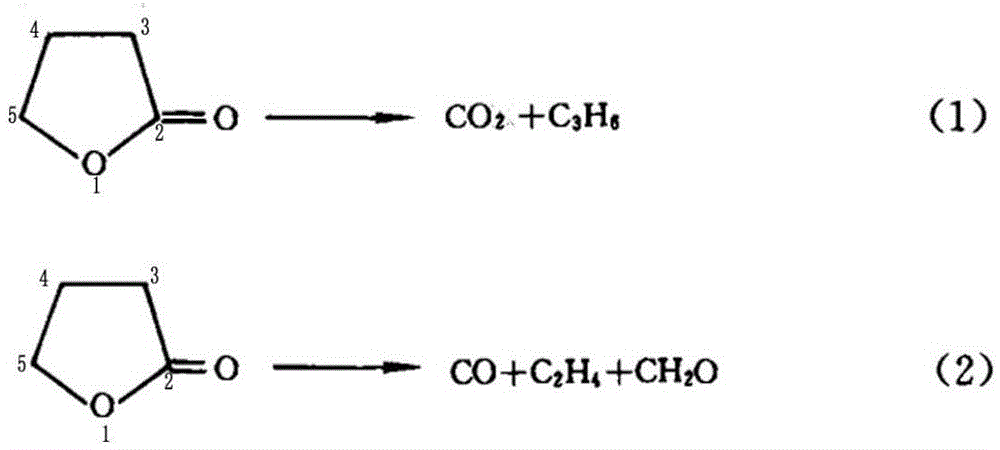
Synthetic method for preparing cyclopropyl methyl ketone by cracking alpha-acetyl-gamma-butyrolactone
ActiveCN109608317ALarge specific surface areaHigh nitrogen contentPreparation from heterocyclic compoundsReaction temperatureKinetic control
The invention discloses a synthetic method for preparing cyclopropyl methyl ketone by cracking alpha-acetyl-gamma-butyrolactone, which comprises the following steps of: adding alpha-acetyl-gamma-butyrolactone material in a venturi-based jet loop reactor, deoxidizing and filling inert gases for replacement and protection, turning on an outside circulating pump for high-speed jet mix, and the like.By the adoption of the synthetic method for preparing the cyclopropyl methyl ketone by cracking the alpha-acetyl-gamma-butyrolactone, a new type of the venture-based jet loop reactor is adopted, so that mass transfer among various reaction species in the reactor is greatly promoted, a reaction is controlled dynamically, and sufficient mass transfer and reaction are achieved between organic phase and catalyst feedstock in a reaction system, the reaction efficiency is greatly improved and occurrence of a side reaction is reduced. Combined catalyst adopted has high catalytic efficiency, high activity and high selectivity, not only reduce the reaction temperature, but also reduce other side reactions of pyrolysis at high temperature, reduce energy consumption and cost.
Owner:JIANGSU YUXIANG CHEM
3,5-dichloro-2-pentanone preparation method
ActiveCN109265329AReduce usageSimple processPreparation from heterocyclic compoundsSulfonyl chlorideOrganic solvent
The invention relates to the field of fine chemicals, and discloses a 3,5-dichloro-2-pentanone preparation method, which comprises: making alpha-acetyl-gamma-butyrolactone contact sulfonyl chloride ina solvent-free system to carry out a chlorination reaction, mixing the material obtained by the chlorination reaction with water, adding hydrochloric acid to the obtained mixture in a dropwise manner, and carrying out a ring-opening reaction. According to the present invention, the solvent-free one-pot reaction is achieved by using the cheap industrial chemicals as the raw materials, such that the process operation is simplified, the three-waste and the production cost are reduced, and the pollution caused by the use of the metal catalyst is avoided.
Owner:NUTRICHEM LAB CO LTD
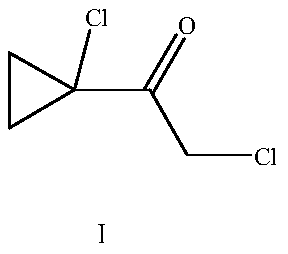
A kind of synthesis technique of 1-chloro-1'-chloroacetyl cyclopropane
ActiveCN104292089BSmooth process connectionReduce unit operationsOrganic compound preparationPreparation from heterocyclic compoundsSulfonyl chlorideChloride
The invention discloses a synthetic process of 1-chloro-cyclopropanecarbonyl chloride, which belongs to the technical field of fine chemical processes. The process is implemented by taking alpha-acetyl-gamma-butyrolactone and sulfonyl chloride as raw materials through the steps of chlorinating, ring cleavage, cyclization, and re-chlorinating, so that a target product is obtained. The synthetic process is implemented by taking cheap industrial chemicals as raw materials and reaction reagents, and by using a single solvent system, a smooth process connection is achieved, so that the reaction yield is improved, the process operation is simplified, the cost of raw materials is reduced, and the cost of production is lowered.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
Method for preparing alpha-acetyl-gamma-butyrolactone by using recycled reaction material
The invention relates to a method for preparing alpha-acetyl-gamma-butyrolactone by using a recycled reaction material, and particularly relates to a method for preparing high-yield alpha-acetyl-gamma-butyrolactone by reaction between recyclable cheap acylating agent and a catalyst in an inert solvent. The method comprises the six steps of acylation reaction, separation of reaction materials, neutral reaction, desolvation and distillation treatment, recovery treatment and cyclic utilization of a waste solvent. Full recycling of the materials is successfully achieved; the problem that a lot of methylbenzene-ethyl ester-ethanol ternary azeotropic solvent is generated in the traditional main technology at home is avoided; all materials can be recycled except for small loss in the reaction process; the environmental pollution is greatly reduced; and the preparation method accords with the national industrial policy at present, and is suitable for industrial popularization.
Owner:HAIZHENG CHEM NANTONG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com