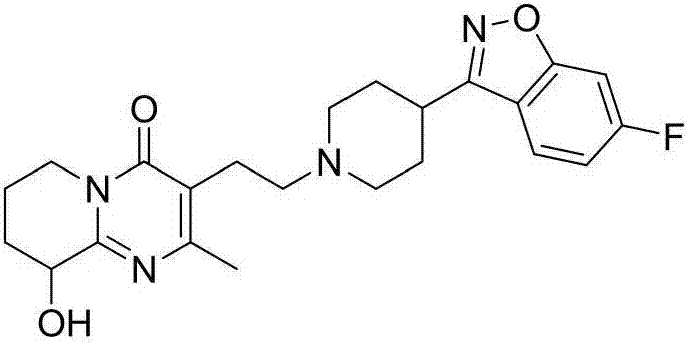
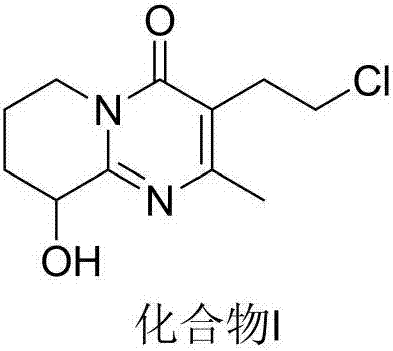
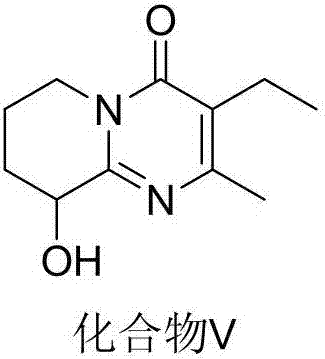
Preparation method of high-purity paliperidone intermediate
A technology of paliperidone and intermediates, applied in the direction of organic chemistry, etc., can solve the problems of poor product properties, cumbersome recrystallization and purification operations, and low product purity, and achieve good properties, shortened hydrogenation reaction time, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of intermediate II: 2-amino-3-benzyloxypyridine
[0036]Control the temperature below 40°C, add 48L of purified water to a 300L stainless steel reaction kettle, slowly add 48kg of sodium hydroxide under stirring conditions, and stir until dissolved. Add 16kg of 2-amino-3-hydroxypyridine, 1.6kg of tetrabutylammonium bromide, and 17.92L of benzyl chloride to the sodium hydroxide solution in sequence, and after the addition, raise the temperature to 70-75°C for 6 hours, stop stirring, and let stand , liquid separation; the water phase was extracted with toluene (20Lx3); the purified water was washed (30Lx2), the organic phase was collected, concentrated until a large amount of solids were precipitated, the temperature was controlled at 0-5°C, stirred and crystallized for 2 hours, centrifuged, and the filter cake was washed with 3.2 Rinse with L pre-cooled toluene, and vacuum dry at 50-55°C for 3 hours to obtain Intermediate II, a bright yellow solid,...
Embodiment 2
[0037] Example 2: Preparation of intermediate III: 9-(benzyloxy)-3-(2-hydroxyethyl)-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
[0038] Under the condition of stirring, add 13.6L toluene, 8.5kg 2-amino-3-benzyloxypyridine, 8.4kgα-acetyl-γ-butyrolactone, 450g p-toluenesulfonic acid successively in the 50L glass reactor, after adding, Raise the temperature to reflux, and separate the water generated by the reaction in time. It is detected by HPLC that the remaining 2-amino-3-benzyloxypyridine is less than 2%. Stop heating, distill under reduced pressure until most of the solids are precipitated, stop the distillation under reduced pressure, and cool down to 0 ~5°C, stirred and crystallized for 2 hours, and filtered with suction to obtain 11.7kg of crude product. Blow dry to constant weight, put the crude product into a 50L reaction kettle filled with 7.7L methanol, heat and reflux for about 1 hour, then cool down to 0-5°C, keep stirring for 1 hour, filter with suction, and blow dr...
Embodiment 3
[0039] Example 3: Preparation of intermediate IV: 9-(benzyloxy)-3-(2-chloroethyl)-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
[0040] Under the condition of stirring at room temperature, 3600ml of ethylene glycol dimethyl ether and 1800g of 9-(benzyloxy)-3-(2-hydroxyethyl)-2-methyl-4H-pyrido[1 ,2-a] pyrimidin-4-one, after uniform dispersion, slowly add 1620ml of phosphorus oxychloride dropwise. After the addition, the temperature was raised to reflux for 6h, the heating was stopped, and the temperature was lowered to room temperature. Slowly add the reaction solution into a 50L glass reaction kettle filled with 7.5L cold water at 0-5°C to quench it. After the addition, a yellow solution is obtained. Use 20% sodium hydroxide solution to adjust the pH to 7-8, and the solution turns brick red. A large amount of solids are produced, continue to stir for 1 hour, filter with suction, wash the filter cake three times (1800mlx3) with purified water at 40-50°C, put the filter cake in an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com