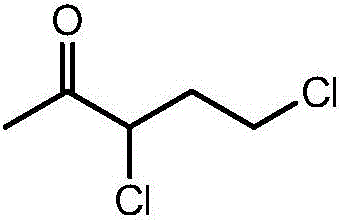
Continuous synthetic method for 3,5-dichloro-2-pentanone
A technology for chemical synthesis and pentanone, which is applied in the field of continuous synthesis of 3,5-dichloro-2-pentanone, can solve the problems of large usage of glacial acetic acid and concentrated hydrochloric acid, cumbersome operation, and poor operating environment. The temperature and feed rate are simple and controllable, the reaction by-products are reduced, and the synthesis cost is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
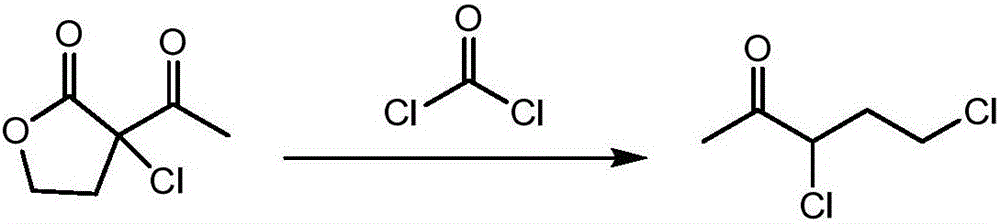
[0028] Dissolve 17.09g (95.10%, 0.10mol) of α-chloro-α-acetyl-γ-butyrolactone in 120.00g of dichloroethane to form α-chloro-α-acetyl-γ-butyrolactone A mixed solution of dichloroethane, and the mixed solution is placed in a raw material bottle with a double plunger pump; open the temperature control system, keep the temperature of the microreactor at 45 ° C, and put the mixed solution in the raw material bottle through a double plunger pump The mixed solution is injected in the microreactor with the feeding speed of 0.5mL / min, simultaneously with 10.10g (98%, 0.10mol) phosgene is directly injected in the microreactor with the rate of 0.0119L / min by gas flowmeter metering, α -Chloro-α-acetyl-γ-butyrolactone and phosgene undergo a continuous chlorination ring-opening reaction in the microreactor, and the reaction solution after the reaction is received at the outlet of the microreactor, and the resulting reaction solution is distilled Finally, 14.81 g of 3,5-dichloro-2-pentanone ...
Embodiment 2
[0030] Dissolve 17.09g (95.10%, 0.10mol) of α-chloro-α-acetyl-γ-butyrolactone in 90.00g of dichloroethane to form α-chloro-α-acetyl-γ-butyrolactone A mixed solution of dichloroethane, and the mixed solution is placed in a raw material bottle with a double plunger pump; open the temperature control system, keep the temperature of the microreactor at 45 ° C, and put the mixed solution in the raw material bottle through a double plunger pump The mixed solution was injected into the microreactor at a feed rate of 2.0mL / min, while 10.10g (98%, 0.10mol) phosgene was metered directly into the reactor by a gas flow meter at a rate of 0.0641L / min, α- Chloro-α-acetyl-γ-butyrolactone and phosgene undergo a continuous chlorination ring-opening reaction in the microreactor, and the reaction solution after the reaction is received at the outlet of the microreactor, and the resulting reaction solution is distilled 14.27 g of 3,5-dichloro-2-pentanone with a mass percentage of 96.83% was obtai...
Embodiment 3
[0032] Dissolve 17.09g (95.10%, 0.10mol) of α-chloro-α-acetyl-γ-butyrolactone in 120.00g of dichloroethane to form α-chloro-α-acetyl-γ-butyrolactone A mixed solution of dichloroethane, and the mixed solution is placed in a raw material bottle with a double plunger pump; open the temperature control system, keep the microreactor at a constant temperature of 30 ° C, and put the mixed solution in the raw material bottle through a double plunger pump The mixed solution was injected into the microreactor at a feed rate of 0.5mL / min, while 11.11g (98%, 0.11mol) phosgene was metered directly into the reactor by a gas flow meter at a rate of 0.0131L / min, α- Chloro-α-acetyl-γ-butyrolactone and phosgene undergo a continuous chlorination ring-opening reaction in the microreactor, and the reaction solution after the reaction is received at the outlet of the microreactor, and the resulting reaction solution is distilled 14.66 g of 3,5-dichloro-2-pentanone with a mass percentage of 97.10% w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com