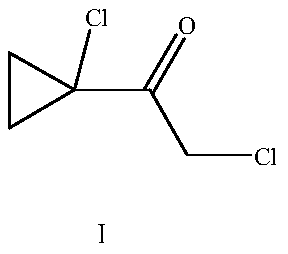
Preparation method of 2-chloro-1-(1-chlorocyclopropyl) acetone
A technology of chlorocyclopropyl and ethyl ketone, which is applied in the field of preparation of 2-chloro-1-ethanone, can solve the problems of long reaction route, long reaction cycle, and low reaction yield, and achieve short reaction route and short reaction cycle. Short, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
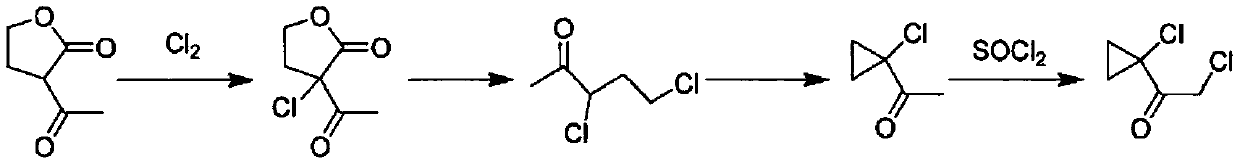
[0030] A preparation method of 2-chloro-1-(1-chlorocyclopropyl)ethanone, comprising the following steps:
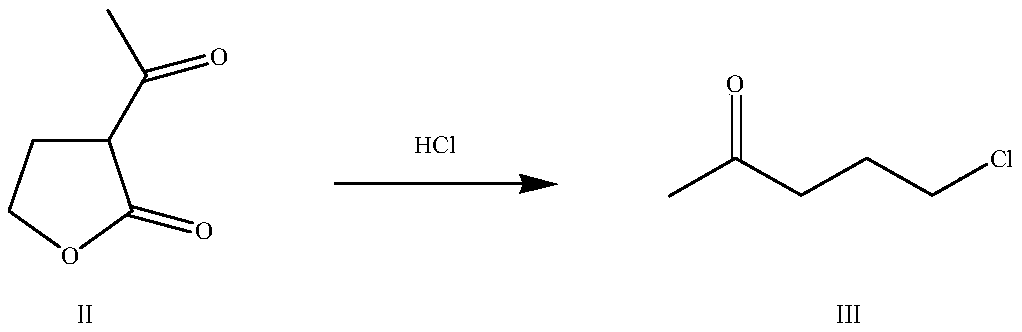
[0031] (1) Add 365.0 g (0.5 mol) of hydrochloric acid with a mass concentration of 5% to a 500 ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a reflux water separator, start stirring, and add α-acetyl-γ-butane dropwise Ester 65.0g (0.5mol), heat up and control the reaction temperature to 40-50°C, carry out the ring-opening decarboxylation reaction for 3h, heat up for distillation, distill water and 5-chloro-2-pentanone azeotropically, and reflux the water to The reaction bottle yielded 55.2 g of 5-chloro-2-pentanone, the gas chromatography analysis content was 96.04%, and the molar yield was 90.60%;
[0032] (2) In the 250ml four-necked flask that mechanical stirring, thermometer, normal pressure distillation unit are housed, add the sodium hydroxide solution 133.3g (0.5mol) that mass fraction is 15%, tetrabutyl ammonium bromide 0.3g, open Stir...
Embodiment 2
[0035] A preparation method of 2-chloro-1-(1-chlorocyclopropyl)ethanone, comprising the following steps:
[0036] (1) Add 365.0 g (1.0 mol) of hydrochloric acid with a mass concentration of 10% into a 500 ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a reflux water separator, start stirring, and add α-acetyl-γ-butyl dropwise Lactone 90.0g (0.7mol), heat up and control the reaction temperature at 50-60°C, react for 2.5h, then heat up for distillation treatment to distill water and 5-chloro-2-pentanone azeotropically, and return the water to the reaction flask , to obtain 79.5 g of 5-chloro-2-pentanone, the gas chromatography analysis content was 95.36%, and the molar yield was 89.82%;
[0037] (2) In the 250ml four-neck flask that mechanical stirring, thermometer, atmospheric distillation unit are housed, adding mass concentration is 20% sodium hydroxide solution 150.9g (0.75mol), tetrabutyl ammonium bromide 0.8g, start stirring , quickly drop 79.5g...
Embodiment 3
[0040] A preparation method of 2-chloro-1-(1-chlorocyclopropyl)ethanone, comprising the following steps:
[0041] (1) Add 486.7g (2.0mol) of hydrochloric acid with a mass concentration of 15% to a 1000ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a reflux water separator, start stirring, and add α-acetyl-γ-butyl dropwise Lactone 128.5g (1.0mol), control the reaction temperature at 60-70°C, react for 1.5h, then heat up and carry out distillation treatment to azeotropically distill water and 5-chloro-2-pentanone, and return the water to the reaction bottle to obtain 5 - Chloro-2-pentanone 114.5g, gas chromatography analysis content 96.18%, molar yield 91.34%;
[0042] (2) In the 500ml four-necked flask that mechanical stirring, thermometer, atmospheric distillation unit are housed, adding mass concentration is 205g (1.28mol) of sodium hydroxide solution of 20%, 2.5g of benzyltriethylammonium chloride, open Stir, quickly add 114.5g of 5-chloro-2-penta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com