Method for preparing alpha-acetyl-gamma-butyrolactone
A technology of butyrolactone and butyrolactone sodium salt, applied in the direction of organic chemistry and the like, can solve the problems of high energy consumption, long purification cycle and high equipment requirements, and achieve the effects of low energy consumption, short purification time and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
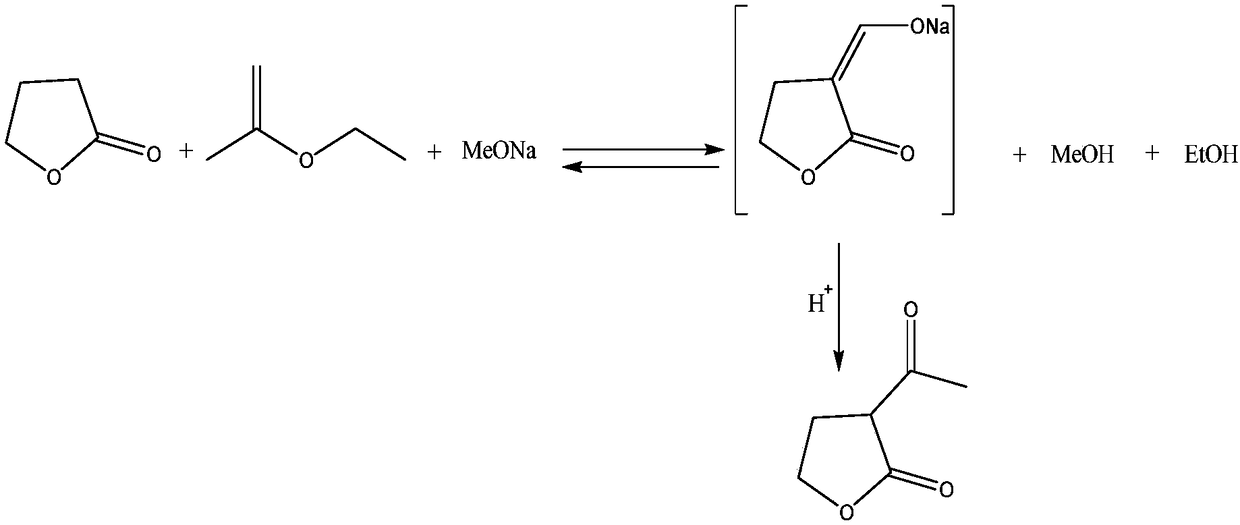
[0017] Add 100g of ethyl acetate into a 500ml reaction flask, heat up to 50°C, and simultaneously add 80g of solid sodium methoxide and the mixture, which is formed by mixing 86g of ethyl acetate and 100g of γ-butyrolactone. Keep the system temperature at 50°C and complete the addition within 2 hours. After the material was added, stirring was continued rapidly at 60° C. for 1 h. For discharging, add the above reaction solution into a 500ml autoclave, and conduct a high-pressure reaction at 100°C for 2 hours. The pressure gauge shows that the pressure is small, and the pressure range is 0.1-1MPa. After the reaction is completed, cool down, discharge, and use 20g of ethyl acetate to wash the reaction Kettle, the washing solution was combined into the reaction solution for use. Transfer the above materials to a 500ml reaction flask, distill under reduced pressure at 50°C (vacuum degree 0.09MPa—0.1MPa), concentrate to near dryness, and the precipitated solid is α-acetyl-γ-butyro...
Embodiment 2
[0020] Add 100g of ethyl acetate into a 500ml reaction flask, heat up to 50°C, add 80g of solid sodium methoxide and the mixed solution at the same time, the mixed solution is formed by mixing 86g of ethyl acetate and 100g of γ-butyrolactone, keep the system temperature at 50°C, Added within 2h. After the material was added, stirring was continued rapidly at 60° C. for 1 h. For discharging, add the above-mentioned liquid materials into a 500ml autoclave, and conduct a high-pressure reaction at 100°C for 2 hours. The pressure gauge shows that the pressure is small, and the pressure range is 0.1-1MPa. After the reaction is completed, cool down, discharge, and use 20g of ethyl acetate to wash the reaction Kettle, the washing solution was combined into the reaction solution for use. The above material was transferred to a 500ml reaction flask, distilled under reduced pressure at 60°C (vacuum degree 0.09MPa—0.1MPa), concentrated to near dryness, and the precipitated solid was α-ac...
Embodiment 3
[0023] Add 100g of ethyl acetate into a 500ml reaction flask, heat up to 50°C, add 80g of solid sodium methoxide and the mixed solution at the same time, the mixed solution is formed by mixing 86g of ethyl acetate and 100g of γ-butyrolactone, keep the system temperature at 50°C, Added within 2h. After the material was added, stirring was continued rapidly at 60° C. for 1 h. For discharging, add the above-mentioned liquid materials into a 500ml autoclave, and conduct a high-pressure reaction at 100°C for 2 hours. The pressure gauge shows that the pressure is small, and the pressure range is 0.1-1MPa. After the reaction is completed, cool down, discharge, and use 20g of ethyl acetate to wash the reaction Kettle, the washing solution was combined into the reaction solution for use. The above materials were transferred to a 500ml reaction flask, distilled under reduced pressure at 60°C (vacuum degree 0.09MPa—0.1MPa), concentrated to near dryness, and α-acetyl-γ-butyrolactone sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com