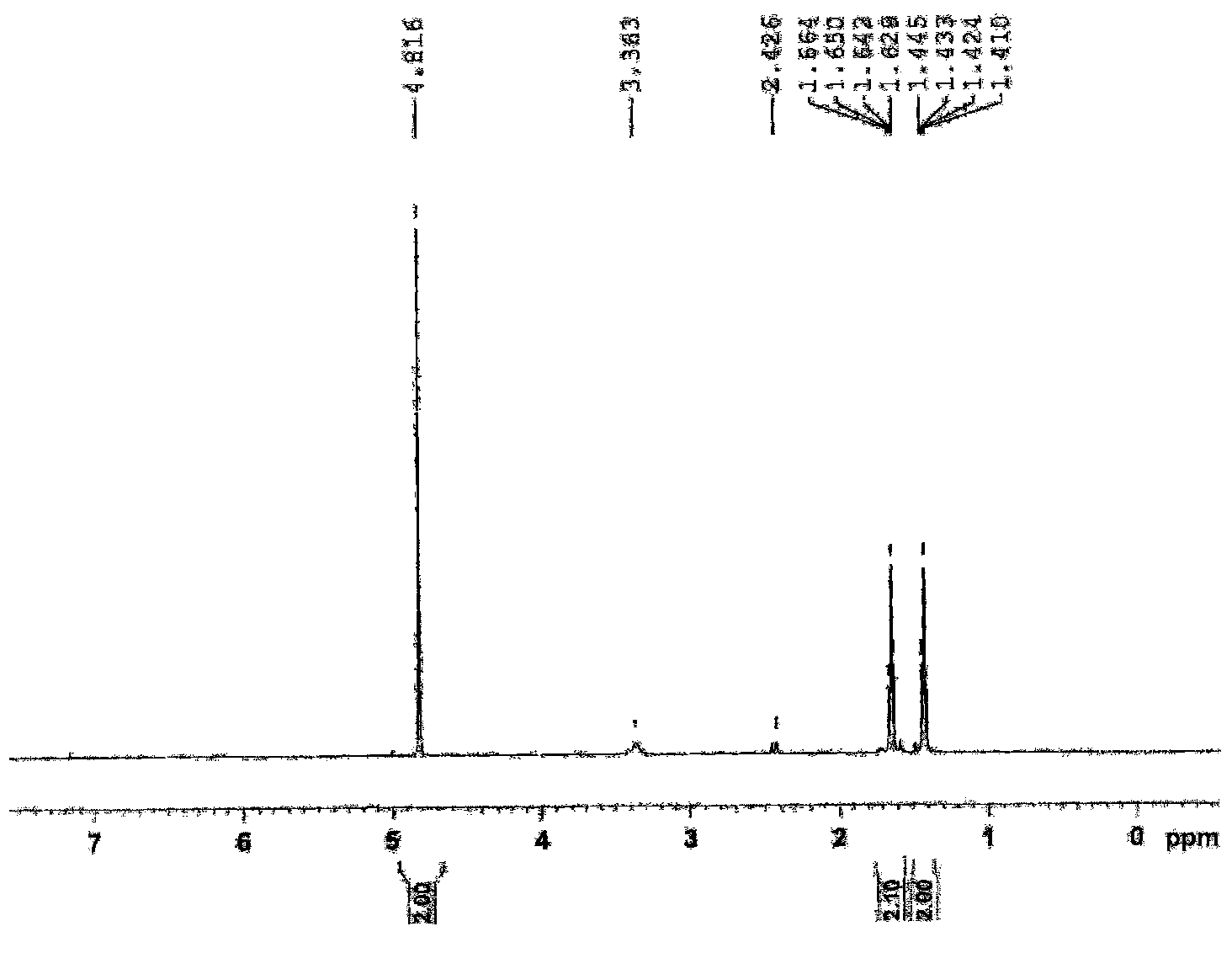
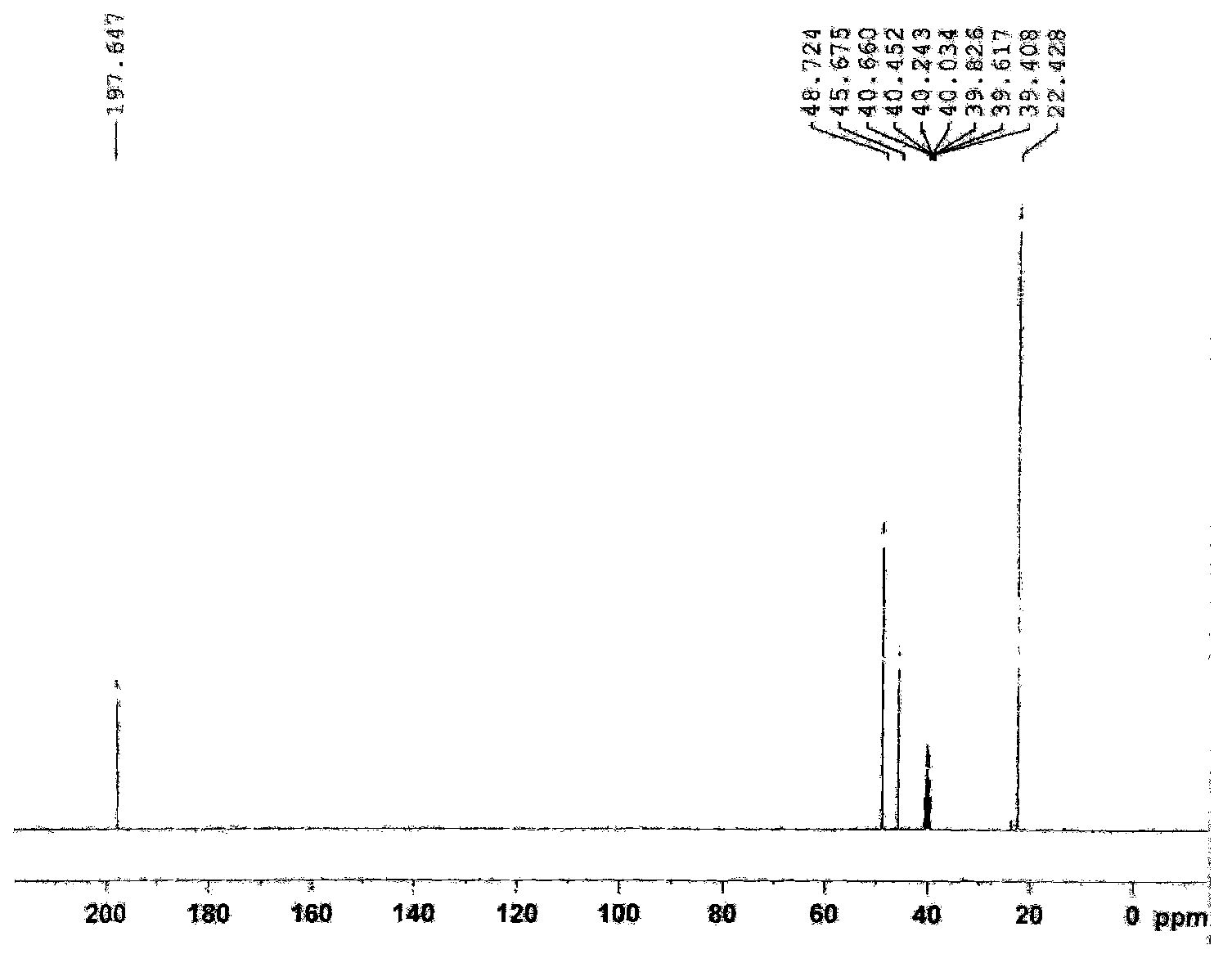
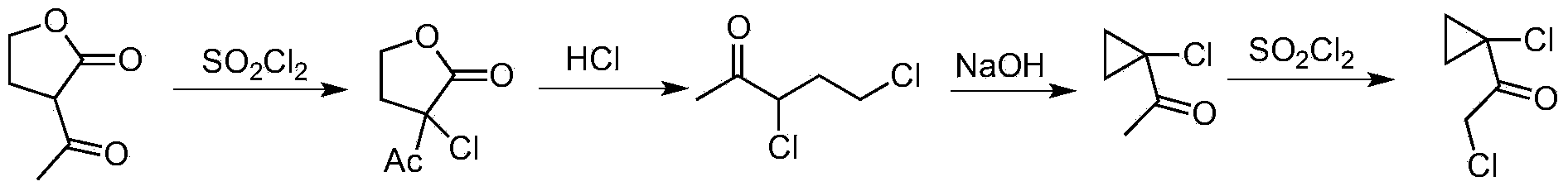
Synthetic process of 1-chloro-cyclopropanecarbonyl chloride
A technology for the synthesis of chloroacetylcyclopropane, which is applied in the field of synthesis of 1-chloro-1'-chloroacetylcyclopropane, can solve the problems of low yield and unsuitability for industrial scale-up production, and achieve simple operation, Typical controllable process conditions and effects of smooth process connections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 1-chloro-1'-chloroacetylcyclopropane.
[0029] (1) Chlorination reaction Ⅰ, synthesis of α-chloro-α’-acetyl-γ-butyrolactone
[0030] Add α-acetyl-γ-butyrolactone 3235.6g (25mol, 99%, 1.0eq) in a 30L jacketed kettle equipped with mechanical stirring, thermometer and tail gas absorption device; cool down to 0°C, start to add sulfuryl chloride dropwise 3408.3g (25mol, 99%, 1.0eq), the temperature control does not exceed 30 ° C, after the dropwise addition is completed, stir for 2 hours until the raw materials are completely converted; after the reaction is completed, remove a small amount of residual sulfuryl chloride, hydrochloric acid gas and sulfur dioxide under reduced pressure to obtain the crude product α -Chloro-α'-acetyl-γ-butyrolactone 4060g yellowish transparent liquid, purity 99.5%, crude yield 100%.
[0031] (2) ring opening reaction, synthesis of 3,5 dichloro-2-pentanone
[0032] Add 4060g α-chloro-α'-acetyl-γ-butyrolactone (25mol, 99.5%, 1.0eq)...
Embodiment 2
[0040] Synthesis of 1-Chloro-1'-Chloroacetylcyclopropane
[0041] The amount of raw materials added in each step and the condition control are the same as in Example 1, and the organic solvent used for extraction in the ring-opening reaction is toluene, so the reaction solvent in other subsequent steps is toluene. The four-step total yield of the product is 101.7%*80%*82%*70%=46.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com