A kind of purification method of anti-IL-17RA monoclonal antibody
A technology of monoclonal antibody and purification method, which is applied in the field of purification of anti-IL-17RA monoclonal antibody, which can solve the problems of complicated antibody purification process, high process purification requirements, and limited administration volume, so as to improve purification efficiency and simplify operation Process, shorten the effect of the purification cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Screening of anti-IL-17RA monoclonal antibodies
[0065] Antibody purification is the most important step in process development. Before the antibody purification method provided by the present invention, anti-IL-17RA monoclonal antibody should be obtained first. The present invention utilizes a fully synthetic ScFv single-chain phage antibody library to screen and obtain a fully human monoclonal antibody specifically binding to IL-17RA. At present, fully human antibodies are the main direction for the development of therapeutic antibodies, and the emergence of antibody library technology provides a good technical platform for the preparation and screening of fully human antibodies. Antibody library technology bypasses the hybridoma process that was necessary in the development of monoclonal antibodies in the past, and can obtain various antibody genes and antibody molecular fragments without even going through the immunization process. Phage antibody library...
Embodiment 2
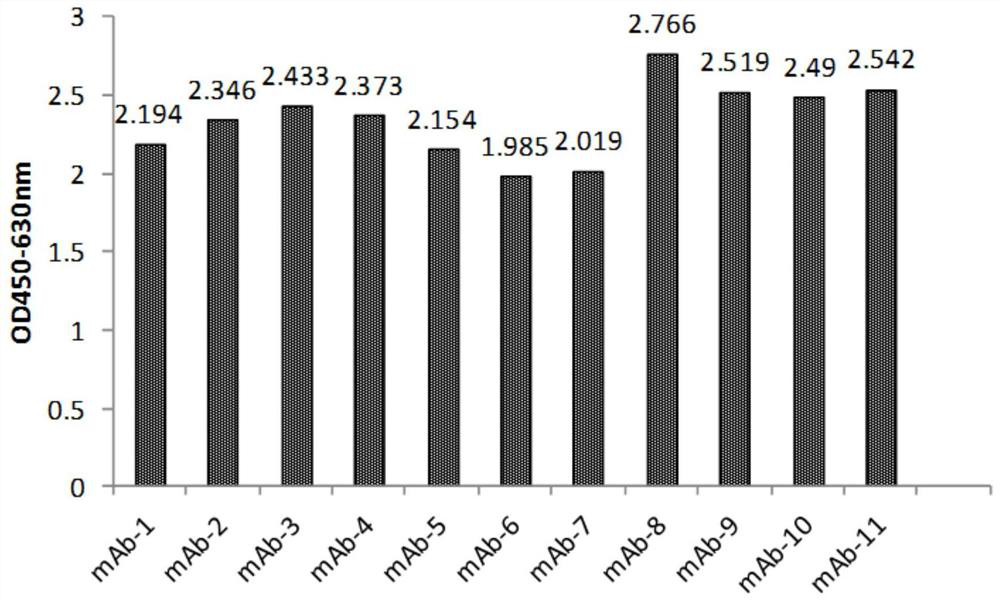
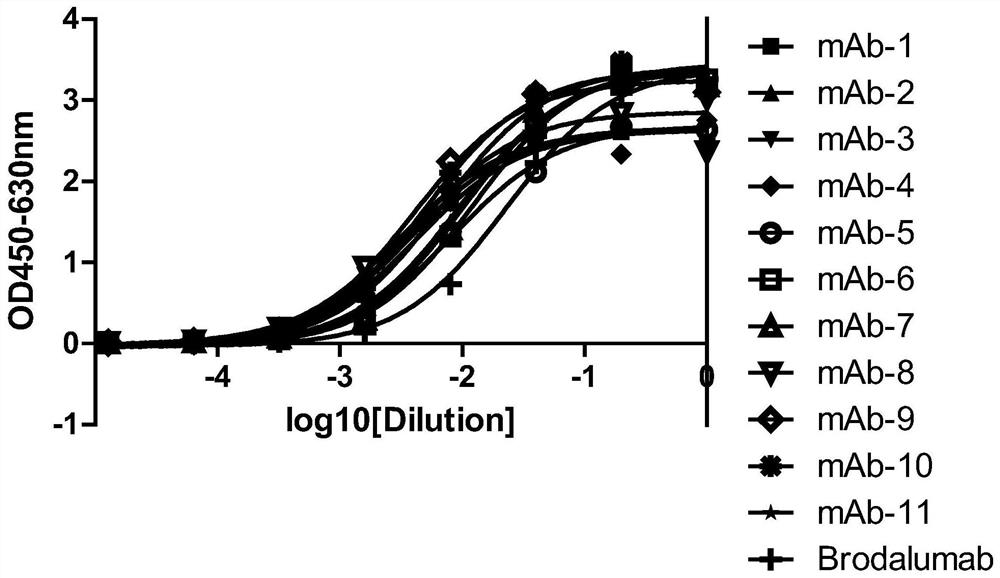
[0113] Example 2, Gradual dilution of phage Elisa to compare the affinity of anti-IL-17RA monoclonal antibodies
[0114] 3.1 Preparation of monoclonal antibody purified phage:
[0115] The 11 monoclonal antibodies (mAb-1, mAb-2, mAb-3, mAb-4, mAb-5, mAb-6, mAb-7, mAb-8, mAb-4, mAb-5, mAb-6, mAb-7, mAb-8, mAb -9, mAb-10, mAb-11) were transferred to 2YTAG liquid medium, shake cultured to the logarithmic growth phase, and then added M13K07 auxiliary phage infection, after centrifugation, the bacteria were resuspended in 2YTAKA, cultured at 28°C overnight and expanded The phages were amplified, and the phages were purified by sedimentation with PEG6000-NaCl the next day. The purified phage of the anti-IL-17RA monoclonal antibody (AMH14 / AML14) provided by the core patent US7833527B2 of the marketed product Brodalumab was used as a positive control.
[0116] 3.2 Affinity comparison at the phage level
[0117] IL-17RA-ECD-His was coated with 0.01M PBS buffer solution of pH 7.2, 10...
Embodiment 3
[0121] Example 3, Preparation of Anti-IL-17RA Whole Antibody
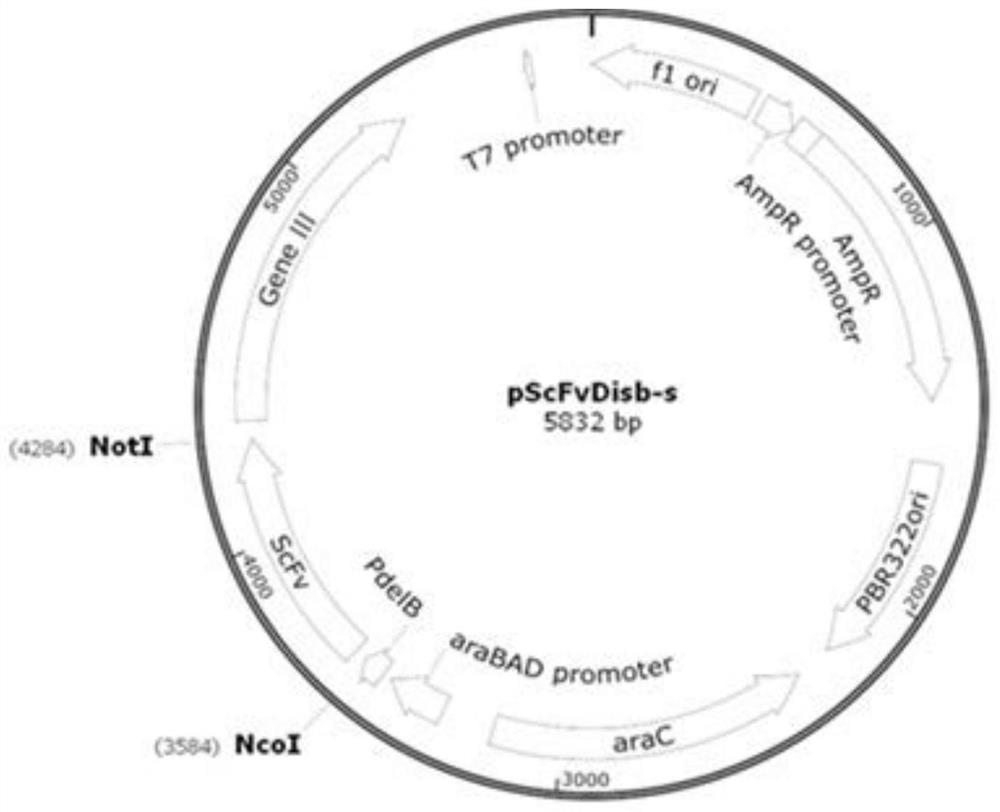
[0122] The heavy chain variable region gene and the light chain variable region gene of the 11 strains of monoclonal antibodies screened in Example 1 were respectively cloned into the vector pTSE containing the heavy chain constant region and the light chain constant region (such as Figure 4 shown), the heavy chain constant region is a human IgG1 constant region (see SEQ ID NO: 14 for the amino acid sequence), the light chain constant region is a κ chain constant region (see SEQ ID NO: 15 for the amino acid sequence), and the vector pTSE is a PTT vector Obtained by basic transformation, the preparation process refers to paragraph [0019] on page 3 of CN103525868A specification, the structure is as follows Figure 4 shown.
[0123] SEQ ID NO: 14 (heavy chain constant region sequence of human IgG1):
[0124] ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com