Method for preparing alpha - acetyl - gamma - butyrolactone
A technology of butyrolactone and acetyl group is applied in the field of preparation of α-acetyl-γ-butyrolactone, which can solve the problems of potential safety hazards, thinning of the wall thickness of the reactor, slow heat dissipation of the reactor, etc., and achieves operational safety. Guarantee, improve quality and yield, shorten the effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
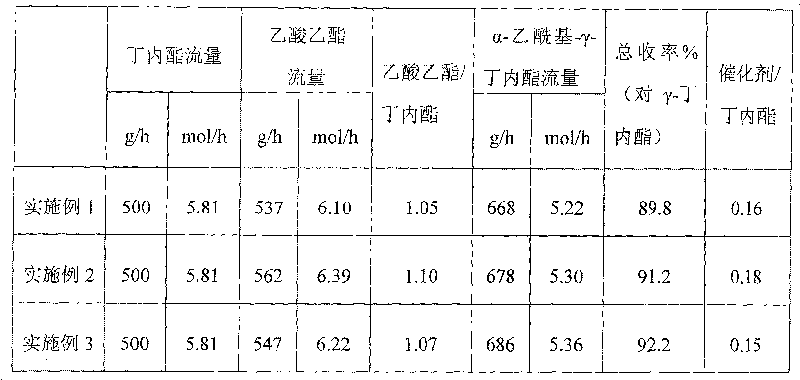
[0020] The feeding flow rate of this embodiment is 500g / h of gamma-butyrolactone and 537g / h of ethyl acetate, and the molar ratio of comprehensive evaluation catalyst and gamma-butyrolactone is 0.16:1. The whole process is divided into a reaction section and a distillation section. The specific operation is as follows:
[0021] The reaction stage is carried out in a fixed-bed reactor equipped with a supported solid catalyst composed of 40% sodium fluoride and 60% composite oxide of silicon dioxide and aluminum oxide, wherein silicon dioxide and aluminum oxide The weight ratio is 1:1, so that the bed temperature starts to feed at 170°C, and the flow rate of γ-butyrolactone 500g / h and ethyl acetate 537g / h is continuously injected into the static mixer and vaporizer through the metering pump , make it vaporized and react in a fixed-bed reactor. The reaction liquid enters the distillation tower after heat exchange. Adjust the reflux ratio device to set the reflux ratio, take samp...
Embodiment 2
[0024] The catalyst of this embodiment is composed of 40% potassium fluoride and 60% composite oxide of silicon dioxide and zinc dioxide, wherein the weight ratio of silicon dioxide and zinc dioxide is 1:1, and the feeding flow rate is γ-butane 500g / h of ester, 562g / h of ethyl acetate are input continuously, other all operate this reaction in the same way as in Example 1, distill to obtain α-acetyl-γ-butyrolactone, its total yield is 91.2%, specific conditions and results See Table 1.
Embodiment 3
[0026] The catalyst of this example is composed of a composite oxide containing 40% potassium fluoride and 60% silicon dioxide and aluminum oxide, wherein the weight ratio of silicon dioxide and aluminum oxide is 1:1, and the feed flow rate is γ - Butyrolactone 500g / h, ethyl acetate 547g / h drop in continuously, other all operate this reaction in the same way as in Example 1, distill to obtain α-acetyl-γ-butyrolactone, and its total yield is 92.2%, specifically See Table 1 for conditions and results.
[0027] Table 1:
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com