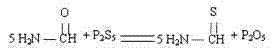Method for synthesizing 4-methyl-5-(2- ethoxy) thiazole
A synthesis method and hydroxyethyl technology, applied in directions such as organic chemistry, can solve the problems of low product quality, complex process steps and high production costs, and achieve the effects of high product quality, simple process steps and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A synthetic method for 4-methyl-5-(2-hydroxyethyl)thiazole, comprising the following steps:
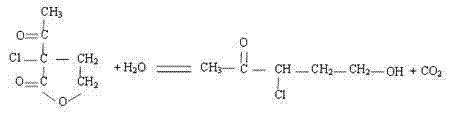
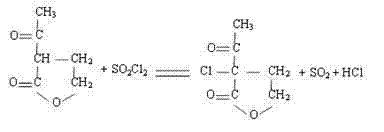
[0025] (1) Oxidation: Add 100 parts by mass of α-acetyl-γ-butyrolactone into a closed reaction kettle with a stirring and cooling device, slowly add 80 parts by mass of sulfonyl chloride dropwise under stirring, and keep the temperature at 40- Under the condition of 42℃, continue to stir for 1.5h, then use CaCl 2 After drying, filter to obtain α-acetyl-α-chloro-γ-butyrolactone;
[0026] (2) Hydrolytic decarboxylation: Take 100 parts by mass of α-acetyl-α-chloro-γ-butyrolactone prepared in step (1), add it to a closed reactor with a stirring and condensing reflux device, and then add 77 parts by mass Parts mass fraction is 5% dilute sulfuric acid or 160 mass parts mass fraction is 5% hydrochloric acid, heated to boiling reflux state, after reacting for 5h, the reaction solution was extracted 3 times with dichloromethane, and the amount of dichloromethane each time was 60 parts...
Embodiment 2
[0030] A synthetic method for 4-methyl-5-(2-hydroxyethyl)thiazole, comprising the following steps:
[0031] (1) Oxidation: Add 100 parts by mass of α-acetyl-γ-butyrolactone into a closed reaction kettle with a stirring and cooling device, slowly add 80 parts by mass of sulfonyl chloride dropwise under stirring, and keep the temperature at 40- Under the condition of 42℃, continue to stir for 2h, then use CaCl 2 After drying, filter to obtain α-acetyl-α-chloro-γ-butyrolactone;
[0032] (2) Hydrolytic decarboxylation: Take 100 parts by mass of α-acetyl-α-chloro-γ-butyrolactone prepared in step (1), add it to a closed reactor with a stirring and condensing reflux device, and then add 77 parts by mass Parts mass fraction is 5% dilute sulfuric acid or 160 mass parts mass fraction is 5% hydrochloric acid, heated to boiling reflux state, after reacting for 7h, the reaction solution was extracted 3 times with dichloromethane, each time dichloromethane consumption was 60 parts by mass...
Embodiment 3
[0036] A synthetic method for 4-methyl-5-(2-hydroxyethyl)thiazole, comprising the following steps:
[0037] (1) Oxidation: Add 100 parts by mass of α-acetyl-γ-butyrolactone into a closed reaction kettle with a stirring and cooling device, slowly add 80 parts by mass of sulfonyl chloride dropwise under stirring, and keep the temperature at 40- Under the condition of 42℃, continue to stir for 2.5h, then use CaCl 2 After drying, filter to obtain α-acetyl-α-chloro-γ-butyrolactone;
[0038] (2) Hydrolytic decarboxylation: Take 100 parts by mass of α-acetyl-α-chloro-γ-butyrolactone prepared in step (1), add it to a closed reactor with a stirring and condensing reflux device, and then add 77 parts by mass Parts mass fraction is 5% dilute sulfuric acid or 160 mass parts mass fraction is 5% hydrochloric acid, heated to boiling reflux state, after reacting for 6h, the reaction solution was extracted 3 times with dichloromethane, each time the amount of dichloromethane was 60 parts by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com