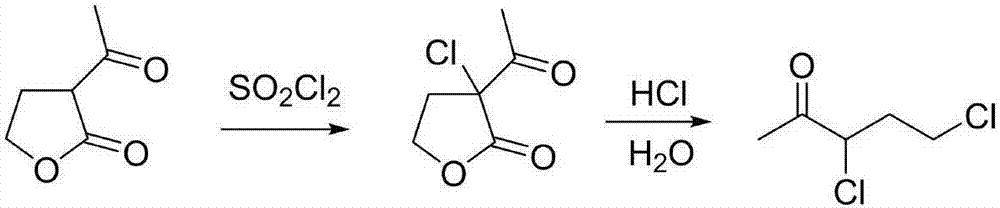
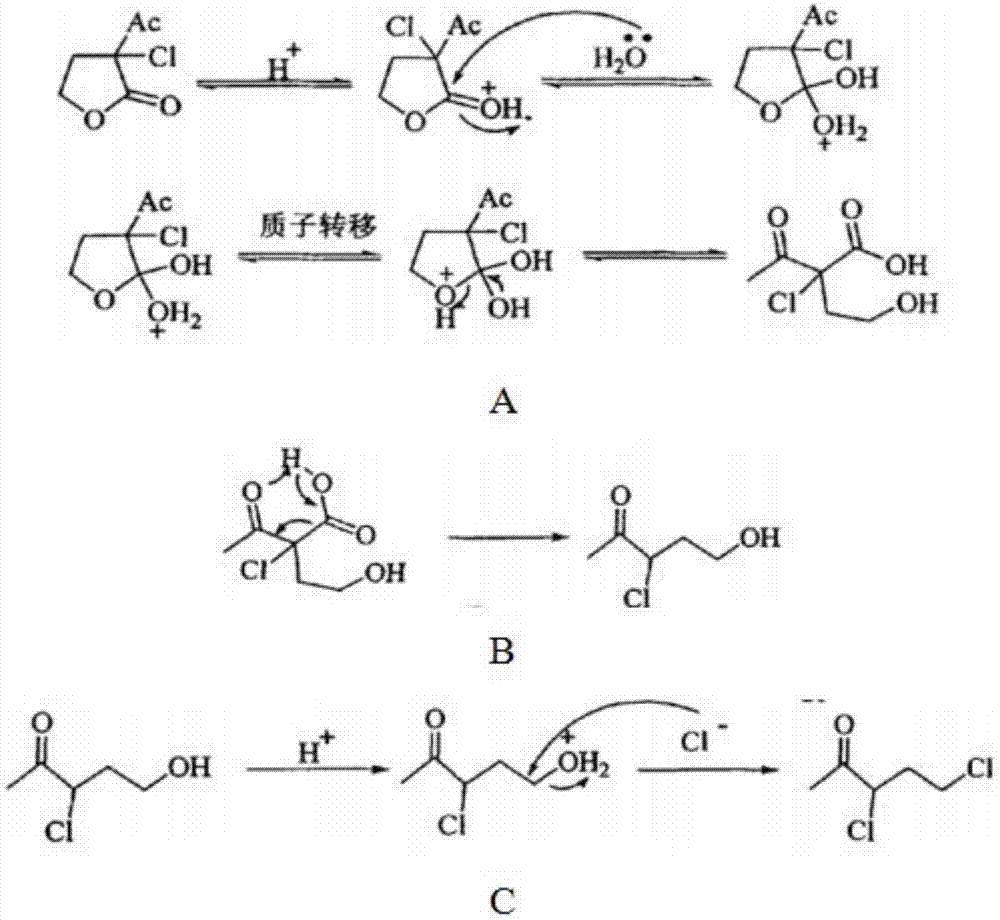
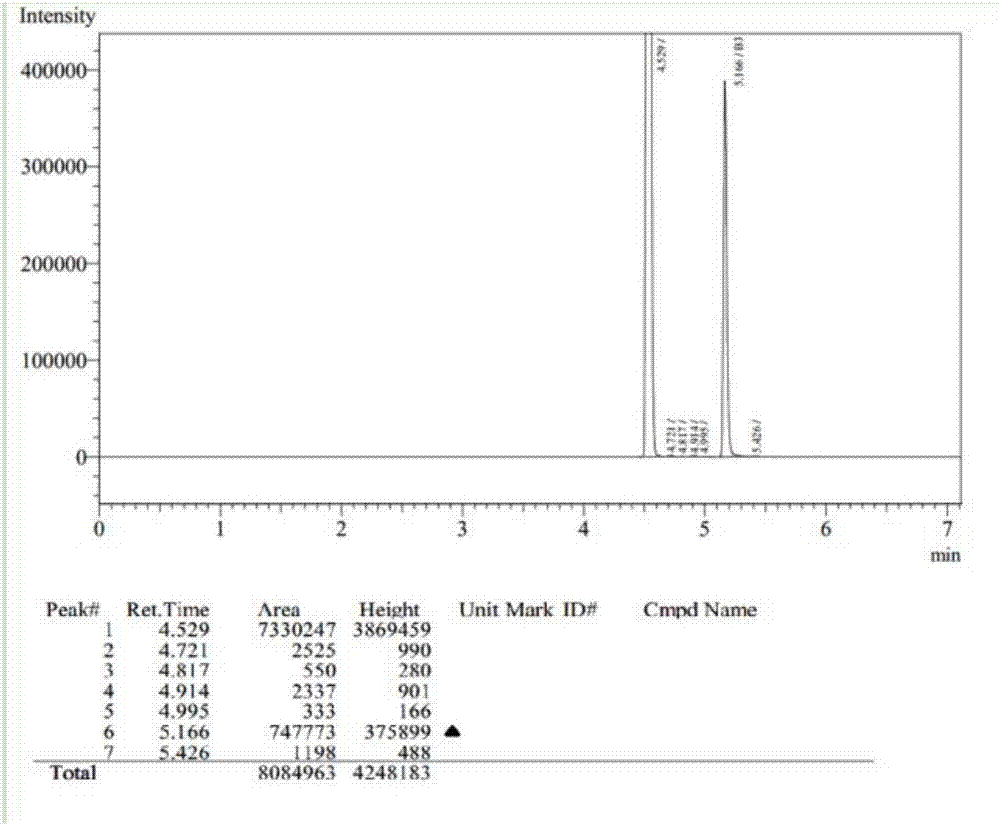
Synthesis process of 3,5-dichloro-2-pentanone
A synthesis process, technology of pentanone, which is applied in the field of synthesis process of 3,5-dichloro-2-pentanone, can solve problems such as impact and unfavorable industrial operation, and achieve increased purity, reduced loss of hydrochloric acid, and less waste Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 9 kg of α-acetyl-γ-butyrolactone into a 20L glass reactor equipped with a stirring device and a thermometer, and cool down the reactor with a circulating cold trap. When the reaction temperature drops to 5°C, start to add 9.9 kg of sulfonyl chloride dropwise , adjust the rate of addition to ensure that the reaction temperature is controlled at about 10°C. After the dropwise addition, the insulation reaction is carried out for 0.5h (to obtain the crude product of α'-chloro-α-acetyl-γ-butyrolactone), and the pressure is reduced at room temperature (-0.1MPa ) and stirred for 2h. Then discharge 11.4kg into a 50L heating kettle, then add 11.4kg of water and 22.8kg of 30% hydrochloric acid in sequence, heat the heat transfer oil to 60°C, keep warm for 0.5h, then heat up to 90°C, keep warm for 0.5h, and continue to heat up to 120°C ℃, heat preservation reaction for 8 hours, the circulating cold trap cools the condenser tube (-15℃), and the azeotrope of 3,5-dichloro-2-penta...
Embodiment 2
[0035] Add 9Kg of α-acetyl-γ-butyrolactone in a 20L glass reactor equipped with a stirring device and a thermometer, and cool down the reactor with a circulating cold trap. When the reaction temperature drops to 10°C, start to add 9.9Kg of sulfuryl chloride dropwise. Control the dropping rate, control the reaction temperature at about 10°C, keep the reaction for 0.5h after the dropwise addition, and stir under reduced pressure (-0.1MPa) at room temperature for 2h. Then put the material into a 50L heating kettle, weigh 11.5Kg, then add 11.5Kg of water and 23Kg of 30% hydrochloric acid in sequence, heat the heat transfer oil to 60°C, keep the temperature for 0.5h, then raise the temperature to 90°C, keep the heat for 0.5h, and continue to heat up To 120°C, hold the reaction for 8 hours, cool down the condenser tube with a circulating cold trap (-15°C), obtain the azeotrope of 3,5-dichloro-2-pentanone and water in the water separator, and directly separate the lower organic phase ...
Embodiment 3
[0037] Add 9Kg of α-acetyl-γ-butyrolactone in a 20L glass reactor equipped with a stirring device and a thermometer, and cool down the reactor with a circulating cold trap. When the reaction temperature drops to 5°C, start to add 9.9Kg of sulfuryl chloride dropwise. Control the dropping rate, control the reaction temperature at about 10°C, keep the reaction for 0.5h after the dropwise addition, and stir under reduced pressure (-0.1MPa) at room temperature for 2h. Then feed into the 50L heating kettle, weigh 11.4Kg, then add 11.4Kg of water, 22.8Kg of 30% hydrochloric acid successively, the heat conduction oil is heated up to 120°C, keep warm for 8h, and the circulating cold trap cools the condenser tube (-15°C), and divides The azeotrope of 3,5-dichloro-2-pentanone and water is obtained in the water tank, and the lower organic phase is directly separated into the product collector to obtain 7.1Kg of high-purity 3,5-dichloro-2-pentanone , The crude yield is 65%, the content is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com