Method and device for synthesizing alpha-chloro-alpha-acetyl-gamma-butyrolactone for co-production of methyl formate
A technology of methyl formate and acetyl, which is applied in the field of synthesizing α-chloro-α-acetyl-γ-butyrolactone to co-produce methyl formate, which can solve the difficulty in the treatment of carbon dioxide and chlorine tail gas and the inability to synthesize hydrochloric acid Utilization, low utilization rate of chlorine gas, etc., to avoid local overheating, improve reaction safety, and avoid excessive chlorine gas concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
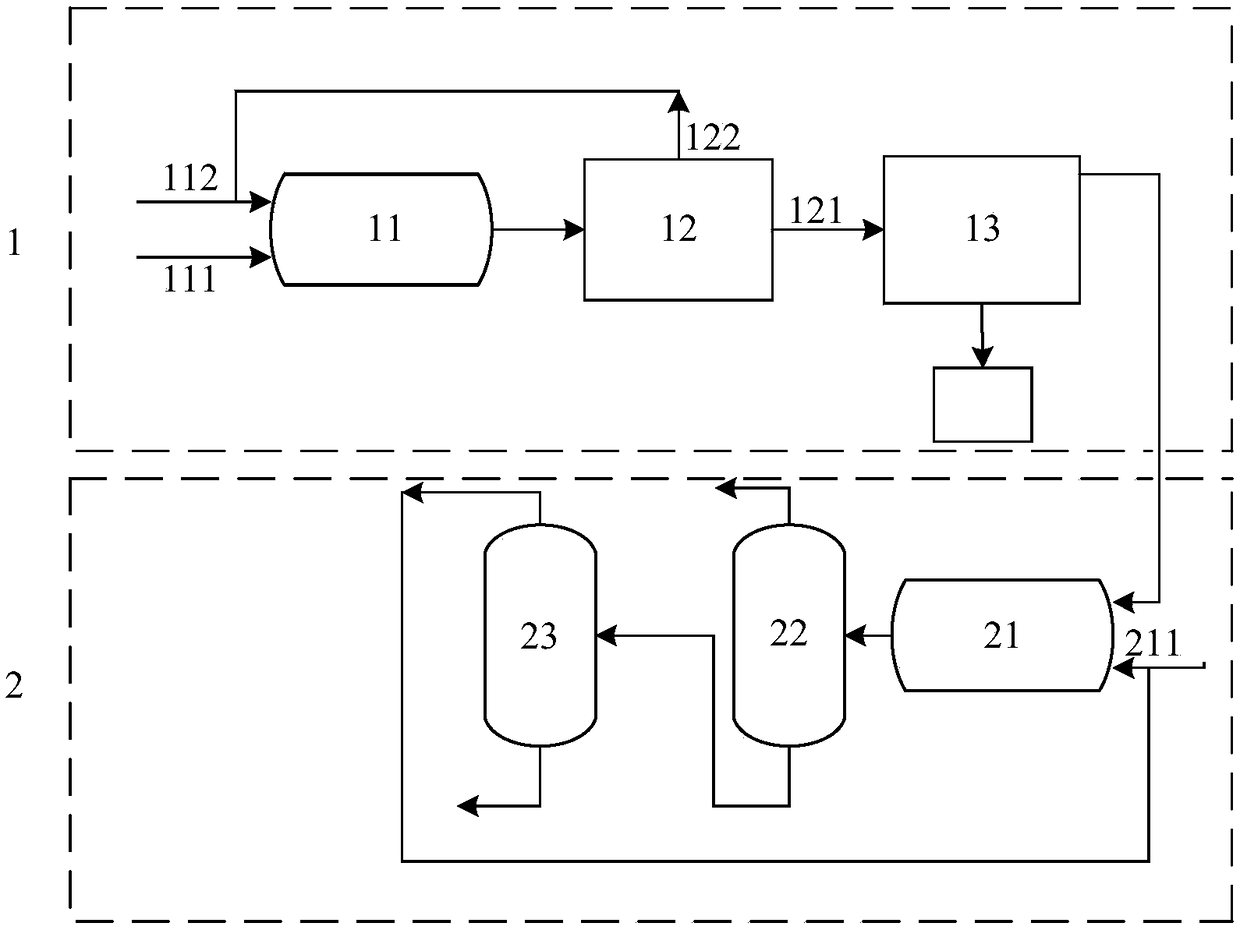
Image
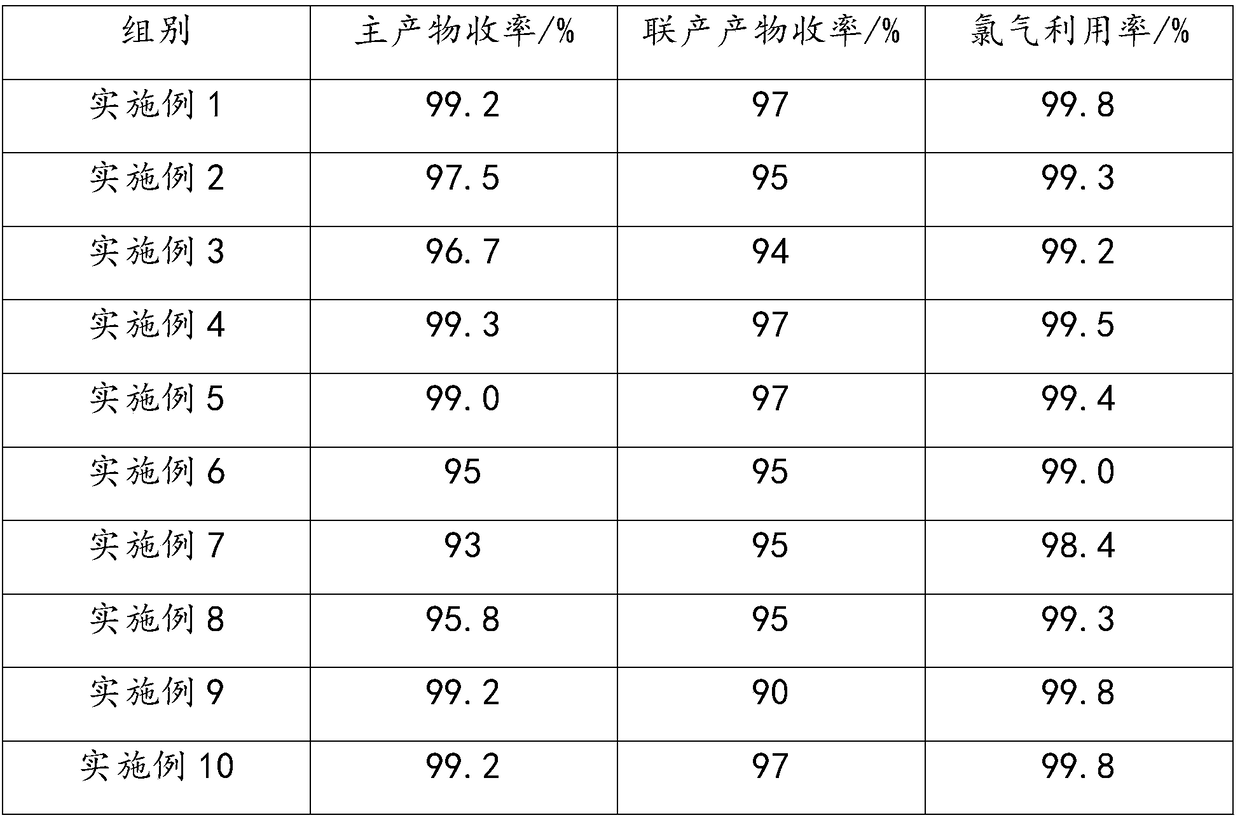
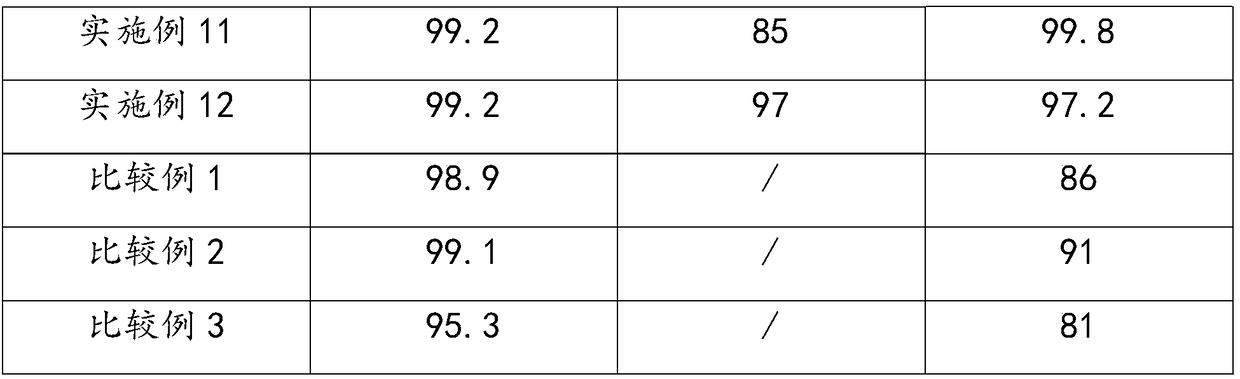
Examples
Embodiment 1
[0065] The method for synthesizing α-chloro-α-acetyl-γ-butyrolactone of the present embodiment to co-produce methyl formate comprises the following steps:
[0066] (1) Get 96g of α-acetyl-γ-butyrolactone, mass fraction of 45% sodium formate aqueous solution 180g, 54g of chlorine continuously into the micropipe reactor, react at 15-16°C, control the reaction of the reactants in the micropipe The residence time in the container is 20-30s. After the reaction, the obtained reaction solution contains the main product α-chloro-α-acetyl-γ-butyrolactone, water, formic acid and sodium chloride;
[0067] (2) The reaction solution after reaction is sent to the flash evaporator from the discharge end of the micropipe reactor, and is directly flashed under the negative pressure condition of -0.05~-0.1MPa, which can separate the slight excess chlorine gas, and after continuous chlorine discharge Obtain the reaction solution 330g after chlorine discharge;
[0068] (3) The reaction solution ...
Embodiment 2
[0072] The method for synthesizing α-chloro-α-acetyl-γ-butyrolactone of the present embodiment to co-produce methyl formate comprises the following steps:
[0073] (1) Get 96g of α-acetyl-γ-butyrolactone, mass fraction of 35% sodium formate aqueous solution 231g, 54g of chlorine continuously into the micropipe reactor, react at 15-16°C, control the reaction of the reactant in the micropipe The residence time in the container is 20-30s. After the reaction, the obtained reaction solution contains the main product α-chloro-α-acetyl-γ-butyrolactone, water, formic acid and sodium chloride;
[0074] (2) After the reaction, the reaction solution is sent to the flash evaporator from the discharge end of the micropipe reactor, and is directly flashed under the negative pressure condition of -0.05Pa~-0.1MPa, which can separate the slight excess chlorine gas and discharge chlorine continuously After obtaining the reaction solution after chlorine discharge;
[0075] (3) The reaction solu...
Embodiment 3
[0079] The method for synthesizing α-chloro-α-acetyl-γ-butyrolactone of the present embodiment to co-produce methyl formate comprises the following steps:
[0080] (1) Get 96g of α-acetyl-γ-butyrolactone, mass fraction of 30% sodium formate aqueous solution 270g, 54g of chlorine continuously into the micropipe reactor, react at 15-16°C, control the reaction of the reactant in the micropipe The residence time in the container is 20-30s. After the reaction, the obtained reaction solution contains the main product α-chloro-α-acetyl-γ-butyrolactone, water, formic acid and sodium chloride;
[0081] (2) The reaction solution after reaction is sent to the flash evaporator from the discharge end of the micropipe reactor, and is directly flashed under the negative pressure condition of -0.05~-0.1MPa, which can separate the slight excess chlorine gas, and after continuous chlorine discharge Obtain the reaction solution after chlorine discharge;
[0082] (3) The reaction solution after th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com