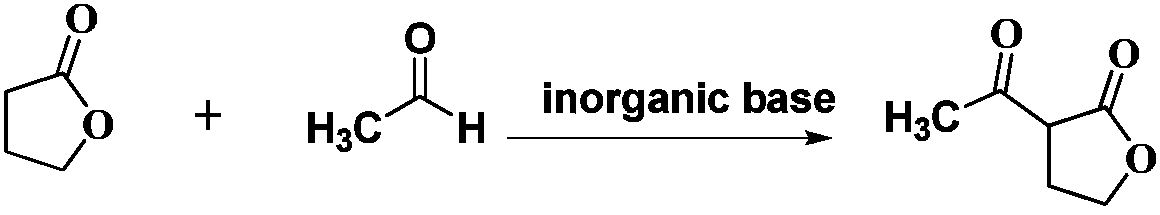Preparation method for Alpha-acetyl-Gamma-butyrolactone
A technology of butyrolactone and acetyl, applied in the field of compound synthesis, can solve the problems of difficult control of feeding speed, easy generation of dust, low cost, etc., and achieves the effects of low equipment selection requirements, suitability for industrialization, and low reaction pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Preparation of α-acetyl-γ-butyrolactone
[0014] Add 150g of isopropyl acetate and 138.2g (1000.0mmol) of potassium carbonate into a 500mL three-necked flask, raise the temperature to 70-74°C, and simultaneously add 43g (500.0mmol) of γ-butyrolactone and the mixed solution, the mixed solution (50g of isoacetic acid Propyl ester and 26.4g (600.0mmol) liquid acetaldehyde mix evenly), control the rate of addition of γ-butyrolactone and mixed solution, guarantee that adding speed is γ-butyrolactone: mixed solution=1.63g:1g (γ-butyrolactone The molar ratio of lactone to acetaldehyde=1:1.2), and the addition was completed in 1h. After adding the materials, continue to stir at 80°C for 30 minutes to remove the gas generated during the reaction. Put the above reaction pressure into a 500mL autoclave, and carry out the acetylation reaction at 94-98°C for 1.5h. After the pressure stabilizes, the range is 0.1-1.0MPa. 20g of isopropyl acetate was used to wash the react...
Embodiment 2
[0016] Example 2: Preparation of α-acetyl-γ-butyrolactone
[0017] Add 100g of ethyl acetate and 63.0g (750.0mmol) of sodium bicarbonate into a 500mL three-necked flask, raise the temperature to 60-65°C, and simultaneously add 43g (500.0mmol) of γ-butyrolactone and the mixture, the mixture (60g of ethyl acetate Esters and 33.0g (750.0mmol) liquid acetaldehyde are mixed evenly), control the drop rate of gamma-butyrolactone and mixed solution, guarantee that adding speed is gamma-butyrolactone: mixed solution=1.95g:1g (gamma-butyrolactone The molar ratio of ester to acetaldehyde=1:1), and the addition was completed in 1h. After the materials were added, the stirring was continued for 30 min at 70°C in order to eliminate the gas generated during the reaction. Put the above reaction pressure into a 500mL autoclave, and carry out the acetylation reaction at 85-90°C for 2 hours. After the pressure stabilizes, the range is 0.1-1.0MPa. Ethyl acetate was used to wash the reactor, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com