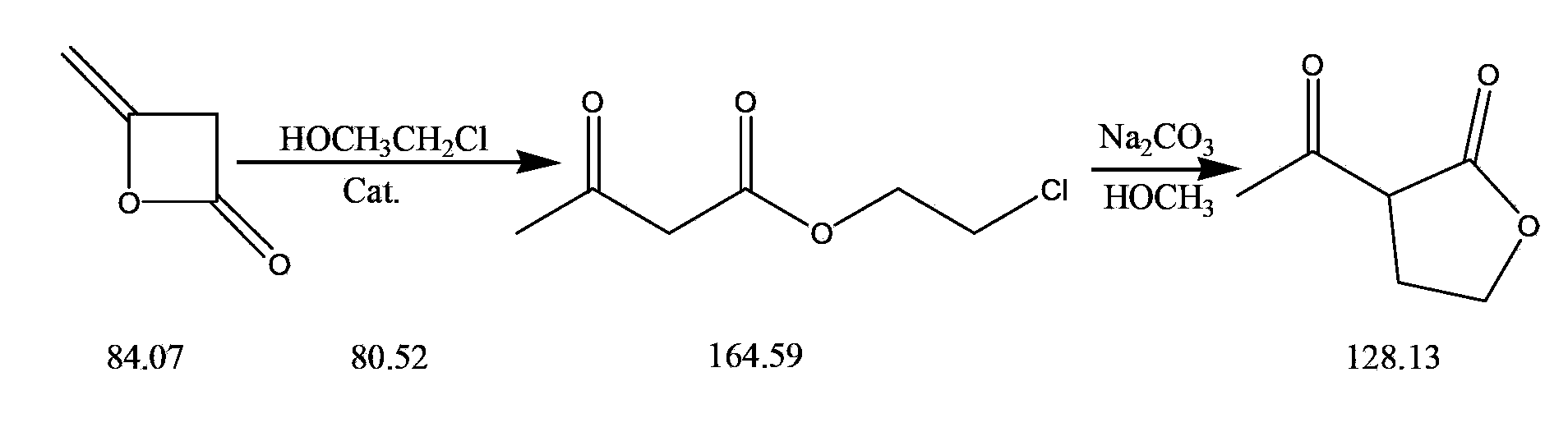
Synthetic method for alpha-acetyl-gamma-butyrolactone
A synthesis method and a technology for butyrolactone, applied in the direction of organic chemistry and the like, can solve the problems of fire, large potential safety hazard, violent reaction, etc., and achieve the effect of reducing potential safety hazard and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of chloroethanol acetoacetate:
[0019] Add 100g of diketene, 105g of 2-chloroethanol, and 20g of sodium ethoxide into a 500ml round-bottomed reaction flask, start stirring, heat to 75°C for reflux reaction for 8 hours, then cool down to room temperature, and the reaction is completed to obtain a mixed solution of 187.09g of chloroethanol acetoacetate (Diketene conversion rate 95%).
[0020] (2) Preparation of α-acetyl-γ-butyrolactone:
[0021] Add 80ml of toluene to the flask containing the mixed solution of chloroethanol acetoacetate, stir and mix and add 175g of 32% liquid caustic soda dropwise at a controlled temperature of 15~30°C, strictly control the reaction temperature (15~30°C), and keep warm for the reaction after the dropwise addition 2h, then use hydrochloric acid to neutralize to PH7.5, continue to stir for 1h, stand to separate layers for 1h, vacuum concentrate the upper layer to a liquid temperature of 120°C, then cool down and release t...
Embodiment 2
[0023] (1) Preparation of bromoethanol acetoacetate:
[0024] Add 100g of diketene, 160g of 2-bromoethanol, and 20g of sodium ethylate into a 500ml round-bottomed reaction flask, start stirring, heat to 75°C for reflux reaction for 8 hours, then cool down to room temperature, and the reaction ends to obtain 236.27g of bromoethanol acetoacetate mixed solution ( The conversion rate of diketene is 95%).
[0025] (2) Preparation of α-acetyl-γ-butyrolactone:
[0026] Add 80ml of toluene to the flask containing the mixed solution of chlorobromoethanol acetoacetate, stir and mix and add 32% liquid caustic soda (175g) dropwise at a controlled temperature of 15~30°C, strictly control the reaction temperature, keep the reaction for 2 hours after the dropwise addition, and then Neutralize to PH7.5 with hydrochloric acid, continue to stir for 1h, let the layers stand for 1h, concentrate the upper layer under reduced pressure to 120°C, then cool down and release the concentrated solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com